Issues Well-Documented
Dodi Froehlich was implanted with an interior vena cava (IVC) filter — a small, spider-shaped medical device designed to stop blood clots from traveling to the heart or lungs — after injuries sustained in a car accident placed her at high risk for clots. Four months later she was in the back of an ambulance, her heart filling with blood and beginning to flat-line.
医生在45岁的Froehlich进行了露天手术,发现一块IVC过滤器已经破坏了设备,前往她的心脏,并刺穿它。
Froehlich survived, but other IVC filter recipients haven’t been so lucky.
格洛丽亚亚当斯,55岁,在患有脑动脉瘤后接受了IVC过滤器。亚当斯在从医院出院后一周死亡。就像Froehlich一样,亚当的心脏被过滤器刺破 - 被血凝块脱落。
Fruehllich和Adams持续的IVC过滤器伤害是与包括C.R. Bard和Cook Medical制造的过滤器相关的模式的一部分。更广泛地,潜在的危险设备亮起利润良好的制造商如何从公众隐瞒产品健康风险以及医疗器械批准过程中的漏洞,可以防止联邦监管机构确保产品安全。
IVC过滤器在肺栓塞治疗中的作用
内部腔静脉是一种大直径静脉,可从身体到心脏的脱氧血液。在开发血栓的人中,腔静脉也可以将凝块携带进入重要的器官,在那里他们有可能导致危及生命的伤害。
抗凝血药物是最常见的血凝凝块治疗,但并非所有患者都是抗凝血剂的好候选者。对于他们来说,IVC过滤器 - 有一个类似的笼状设计,使静脉中的血凝块 - 可能是更安全的处理。
Filters are either retrievable (meant to be removed from the body when blood clot risks subside) or non-retrievable (permanent). Problems with the devices that have emerged in recent years center on the retrievable types.
A Pattern of Harm
放置在主要血液途径中的小型器件具有危险副作用的固有风险。除了出血和感染等并发症外,设备本身的故障都与大量不良事件有关。
2005年至2010年间,FDA收到了近1,000个IVC过滤器不良事件报告。报告的最严重事件是设备迁移(328患者投诉),设备组件的分离(146份投诉),IVC穿孔(70份投诉)和过滤骨折(56份投诉)。
They relayed this information in an August 2010 communication which stated that, “These types of events may be related to a retrievable filter remaining in the body for long periods of time, beyond the time when the risk of pulmonary embolism has subsided”.
建议尽可能注意,只要不再需要肺栓塞保护,就会从患者中移除IVC过滤器。
在2014年5月发布的后续沟通中,FDA重申了这一建议。他们还指出,整个设备可以迁移(类似于Gloria Adams的内容),并且可以难以删除该设备。他们建议医生评估设备去除对每位患者的风险和益处 - 这意味着在某些情况下,可以不必要地危险。
这些建议是基于最近公布的研究和邮政市场监测研究。FDA未引用的一项研究(2013年在JAMA发布)发现,少于10%的IVC过滤器已从患者中成功删除,而8%的过滤器受体仍然是肺栓塞,尽管该装置遭受了肺栓塞。伴随着Jama研究的伴侣结论是,“IVC过滤器应在达到肺循环之前捕获血凝块,并应改善结果。然而,这种理论从未通过实证研究验证“。
伴侣作者继续使用IVC过滤器来说明“美国医疗器械批准的缺点”。
FDA的510(k)批准过程
大多数患者假设医生规定的药物和医疗器械在临床测试中被确定为安全有效。虽然这是大多数药物和设备的情况,但它在董事会上不是真实的。
作为预算有限的联邦机构,FDA没有所需的资源需要对公司希望市场上市的每种新药物和设备进行自己的临床测试。制造商开展自己的临床测试,FDA评论测试数据,以确保它建立了产品安全性和功效。
Product safety is not held to an absolute standard because if new products were expected to produce no harm to any patient under all circumstances, beneficial drugs and devices would almost never make it to market. To promote innovation, the FDA also allows fast-track approval of some new medical devices under what is known as the “510(k)” process. This process allows new medical devices to forego clinical testing if they are said to be “substantially equivalent” to a device already on the market.
510(k)进程受到医学专家的严重批评 - 有些人呼吁它破碎超越维修。在追求的新英格兰医学杂志中,医生David R.挑战者和William W.Vodra描述了目前市场上的设备如何追溯其“大量等价”返回所有临床意义的地点:
Today, we have a system in which a new moderate-risk device can enter the market because it is substantially equivalent to another device that may have been cleared for marketing 2 years ago because its manufacturer showed that it was substantially equivalent to yet another device cleared in 2003, and so on, all the way back to a device that was being marketed when the law was enacted in 1976. But that original device might never have been assessed for safety or effectiveness, nor perhaps would any subsequent ones in the family tree.
About 1/3 of all new medical devices sold in the United States are approved through the 510(k) process. Some of these have caused widespread patient harm, including the infamous DePuy ASR metal hips which had a failure rate approaching 50% and caused severe injuries such as metal poisoning, hip dislocation, and bone fractures.
Bard Ivc恢复
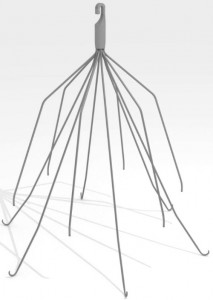
另外510(k)批准的设备是BARD恢复IVC过滤器 - 相同的过滤器负责Gloria Adams死亡以及Dodi Froehlich的近乎死亡。恢复IVC与至少27人死亡和300多人伤害有关,并且失败率为16%。
吟游诗人在2002年提交的第一次尝试时没有成功于510(k)批准,以2002年提交。作为回应,Bard聘请监管专家Kay Resour y帮助批准过程。富勒对恢复的安全表示担忧。具体来说,她说,吟呦诗人从她身上扣留了重要的安全证据,并且她所看到的证据是令人不安的。当富勒表达她的担忧时,她说吟呦诗人威胁要解雇她的团队。尽管她拒绝签署设备的FDA申请,但富勒的签名出现在文件上 - 这是一个明显的伪造。
Fuller expressed her concerns to the FDA but the agency has yet to formally address them. They did, however, send a warning letter to Bard in July 2015 reprimanding the manufacturer for failing to properly establish and maintain procedures for reviewing and evaluating complaints relating to its G2, G2X and Eclipse IVC filters.
The Bard G2 IVC filter was also approved under the 510(k) approval process after it was deemed to be substantially equivalent to the problematic Recovery IVC filter. The G2 filter has been shown to have a 12% failure rate.
通过510(k)工艺批准的其他IVC过滤器包括厨师Günther郁金香腔静脉滤波器和Cook®Celect®Vena静脉过滤器。在2012年的研究中发现了这些库克IVC,以具有100%的腔静脉穿孔率和40%的设备倾斜率。
Bard May Have Withheld Risks From Public
证据表明,Bard知道恢复过滤器的危险,但未能与公众分享此信息。
证据来自Bard委托并由NBC新闻获得的报告。它表明,随着2004年,设备制造商可能知道,与其所有竞争对手相比,恢复更容易骨折,迁移和引起患者死亡。但是Bard未能提供召回。它销售了3年的设备,其中植入了34,000名患者。然后吟游诗人修改,重命名和再培营设备作为G2 - 植入估计的62,000名患者。
Victims File Lawsuits Against Filter Makers
Since suffering a punctured heart that almost killed her, Dodi Froehlich has filed a lawsuit against C.R. Bard, Inc. She is joined by dozens of other device recipients who allege to also have suffered injuries caused by their Bard IVC filters.
所有BARD IVC筛选索赔的多渠道诉讼(MDL)目前在亚利桑那州美国地区法院巩固。截至2015年9月15日,MDL包含50个单独的伤害索赔。
Cook Medical is also dealing with an IVC MDL consolidated in Southern Indiana U.S. District Court. The Cook MDL currently has 120 claims. Similar to the Bard IVC MDL, the Cook MDL is related to the alleged ability of Cook IVC filters to cause injuries when they fracture, migrate, tilt, and perforate.
Sources
- http://www.fda.gov/safety/medwatch/safetyinformation/safyeterertsforhumanMedical产品产品/ ucm221707.htm
- http://www.nbcnews.com/health/health-news/did-blood-clot-filter-used-thousands-americans-have-fatal-flaw-n384536
- http://archinte.jamanetwork.com/article.aspx?articled=1669107
- http://www.medscape.com/viewarticle/744331_2
- http://www.nejm.org/doi/full/10.1056/nejmp1109150?viewtype=print&

