FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
热门话题
Tarceva Recall
获取警报when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Enter section text here
TARCEVA单药治疗用于治疗至少一种先前的化疗方案失败后局部晚期或转移性非小细胞肺癌的患者[见临床研究(14.1)]。
Results from two, multicenter, placebo-controlled, randomized, Phase 3 trials conducted in first-line patients with locally advanced or metastatic NSCLC showed no clinical benefit with the concurrent administration of TARCEVA with platinum-based chemotherapy [carboplatin and paclitaxel or gemcitabine and cisplatin] and its use is not recommended in that setting [see Clinical Studies (14.3)].
TARCEVA in combination with gemcitabine is indicated for the first-line treatment of patients with locally advanced, unresectable or metastatic pancreatic cancer [see Clinical Studies (14.3)].
History
There is currently no drug history available for this drug.
Other Information
TARCEVA (erlotinib), a kinase inhibitor, is a quinazolinamine with the chemical name N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine. TARCEVA contains erlotinib as the hydrochloride salt that has the following structural formula:
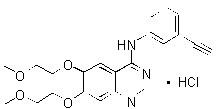
Erlotinib hydrochloride has the molecular formula C22H23N3O4.hcl和分子量为429.90。该分子有PKaof 5.42 at 25oC. Erlotinib hydrochloride is very slightly soluble in water, slightly soluble in methanol and practically insoluble in acetonitrile, acetone, ethyl acetate and hexane.
盐酸厄洛替尼的水溶性取决于pH,由于二级胺的质子化,pH值的溶解度增加在pH值小于5时的溶解度。在1.4至9.6的pH范围内,最大溶解度约为0.4 mg/mL,在pH下发生约2。
TARCEVA tablets for oral administration are available in three dosage strengths containing erlotinib hydrochloride (27.3 mg, 109.3 mg and 163.9 mg) equivalent to 25 mg, 100 mg and 150 mg erlotinib and the following inactive ingredients: lactose monohydrate, hypromellose, hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, sodium lauryl sulfate and titanium dioxide. The tablets also contain trace amounts of color additives, including FD&C Yellow #6 (25 mg only) for product identification.
Sources
Tarceva Manufacturers
-
医生总保健公司。
![Tarceva(盐酸埃洛替尼)片剂[医师Care,Inc。]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Tarceva |医生总保健公司。
![Tarceva (Erlotinib Hydrochloride) Tablet [Physicians Total Care, Inc.] Tarceva(盐酸埃洛替尼)片剂[医师Care,Inc。]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Enter section text here
2.1 Recommended Dose - NSCLCThe recommended daily dose of TARCEVA for non-small cell lung cancer is 150 mg taken at least one hour before or two hours after the ingestion of food. Treatment should continue until disease progression or unacceptable toxicity occurs. There is no evidence that treatment beyond progression is beneficial.
2.2 Recommended Dose - Pancreatic CancerThe recommended daily dose of TARCEVA for pancreatic cancer is 100 mg taken at least one hour before or two hours after the ingestion of food, in combination with gemcitabine (see the gemcitabine package insert). Treatment should continue until disease progression or unacceptable toxicity occurs.
2.3剂量修饰In patients who develop an acute onset of new or progressive pulmonary symptoms, such as dyspnea, cough or fever, treatment with TARCEVA should be interrupted pending diagnostic evaluation. If Interstitial Lung Disease (ILD) is diagnosed, TARCEVA should be discontinued and appropriate treatment instituted as necessary [see Warnings and Precautions (5.1)].
洛派丁胺腹泻通常可以管理。Patients with severe diarrhea who are unresponsive to loperamide or who become dehydrated may require dose reduction or temporary interruption of therapy. Patients with severe skin reactions may also require dose reduction or temporary interruption of therapy.
当剂量减少necessary, the TARCEVA dose should be reduced in 50 mg decrements.
In patients who are taking TARCEVA with a strong CYP3A4 inhibitor such as, but not limited to, atazanavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, troleandomycin (TAO), voriconazole, or grapefruit or grapefruit果汁,如果发生严重的不良反应,应考虑减少剂量。同样,在用CYP3A4和CYP1A2抑制剂(如环丙沙星)服用Tarceva的患者中,如果发生严重的不良反应,应考虑降低Tarceva的剂量。[参见药物相互作用(7)]。
与CYP3A4诱导蛋白利福平的预处理减少了约2/3至4/5。强烈建议使用缺乏CYP3A4诱导活性的替代处理。如果无法获得替代治疗,则应将Tarceva剂量的增加视为在监测患者安全性的同时,在两周内耐受。与利福平结合研究的Tarceva的最大剂量为450 mg。如果将TARCEVA剂量向上调节,则在停用利福平或其他诱导剂后,需要立即将剂量立即减少到指定的起始剂量。其他CYP3A4诱导剂包括但不限于利法布丁,利法帕汀,苯妥英钠,卡马西平,苯巴比妥和圣约翰麦芽汁。如果可能的话,也应避免这些[请参见药物相互作用(7)]。
Cigarette smoking has been shown to reduce erlotinib exposure. Patients should be advised to stop smoking. If a patient continues to smoke, a cautious increase in the dose of TARCEVA, not exceeding 300 mg may be considered, while monitoring the patient’s safety. However, efficacy and long-term safety (> 14 days) of a dose higher than the recommended starting doses have not been established in patients who continue to smoke cigarettes. If the TARCEVA dose is adjusted upward, the dose should be reduced immediately to the indicated starting dose upon cessation of smoking [see Clinical Pharmacology (12.3)].
Erlotinib is eliminated by hepatic metabolism and biliary excretion. Although erlotinib exposure was similar in patients with moderately impaired hepatic function (Child-Pugh B), patients with hepatic impairment (total bilirubin > ULN or Child-Pugh A, B and C) should be closely monitored during therapy with TARCEVA [see WARNINGS and PRECAUTIONS (5.2 )]. Treatment with TARCEVA should be used with extra caution in patients with total bilirubin > 3 x ULN. TARCEVA dosing should be interrupted or discontinued if changes in liver function are severe such as doubling of total bilirubin and/or tripling of transaminases in the setting of pretreatment values outside normal range. In the setting of worsening liver function tests, before they become severe, dose interruption and/or dose reduction with frequent liver function test monitoring should be considered. TARCEVA dosing should be interrupted or discontinued if total bilirubin is >3 x ULN and/or transaminases are >5 x ULN in the setting of normal pretreatment values [see Warnings and Precautions (5.2 , 5.3 ), Adverse Reactions (6.3) and Use in Specific Populations (8.6 )].
-
Genentech,Inc。
![Tarceva (Erlotinib Hydrochloride) Tablet [Genentech, Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Tarceva |Genentech,Inc。
![Tarceva (Erlotinib Hydrochloride) Tablet [Genentech, Inc.] Tarceva (Erlotinib Hydrochloride) Tablet [Genentech, Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Patient SelectionSelect patients for the first-line treatment of metastatic NSCLC with TARCEVA based on the presence of EGFR exon 19 deletions or exon 21 (L858R) substitution mutations in tumor specimens [See Clinical Studies (14.1)]. Information on FDA-approved tests for the detection of EGFR mutations in NSCLC is available at: http://www.fda.gov/CompanionDiagnostics
2.2 Recommended Dose – NSCLCThe recommended daily dose of TARCEVA for NSCLC is 150 mg taken on an empty stomach, i.e., at least one hour before or two hours after the ingestion of food. Treatment should continue until disease progression or unacceptable toxicity occurs.
2.3 Recommended Dose – Pancreatic CancerThe recommended daily dose of TARCEVA for pancreatic cancer is 100 mg taken once daily in combination with gemcitabine. Take TARCEVA on an empty stomach, i.e., at least one hour before or two hours after the ingestion of food. Treatment should continue until disease progression or unacceptable toxicity occurs [see Clinical Studies (14.5)].
2.4 Dose ModificationsDiscontinue TARCEVA for:
• Interstitial Lung Disease (ILD) [see Warnings and Precautions (5.1)]. • Severe hepatic toxicity that does not improve significantly or resolve within three weeks [see Warnings and Precautions (5.3)]. • Gastrointestinal perforation [see Warnings and Precautions (5.4)]. • Severe bullous, blistering or exfoliating skin conditions [see Warnings and Precautions (5.5)]. • Corneal perforation or severe ulceration [see Warnings and Precautions (5.9)].Withhold TARCEVA:
•在可能的ILD诊断评估期间。•对于严重的(CTCAE 3至4级)肾脏毒性,并考虑停用Tarceva [请参阅警告和预防措施(5.2)]。•在没有预先存在的肝损伤的患者中,总胆红素水平大于正常或转氨酶上限的3倍大于正常上限的5倍,并考虑停用TARCEVA [请参见警告和预防措施(5.3)]。•对于抗肝损伤或胆道障碍物的患者,胆红素增加了一倍或跨基线的三倍倍跨基线,并考虑停用TARCEVA [请参阅警告和预防措施(5.3)]。•对于持续的严重腹泻,对医疗管理的反应(例如,洛哌丁胺)。•严重皮疹对医疗管理的反应。•对于(NCI-CTC 4.0版)3-4级或2级持续2周的角膜炎[请参见警告和预防措施(5.9)]。•对于急性/恶化的眼部疾病,例如眼痛,并考虑停用Tarceva [请参阅警告和预防措施(5.9)]。Reduce TARCEVA by 50 mg decrements:
• If severe reactions occur with concomitant use of strong CYP3A4 inhibitors [such as atazanavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, troleandomycin (TAO), voriconazole, or grapefruit or grapefruit juice] or when同时使用CYP3A4和CYP1A2(例如环丙沙星)的抑制剂。如果可能的话,请避免使用[请参阅药物相互作用(7)]。•在预扣治疗后,对剂量限制毒性进行预扣治疗后,该治疗方法已决定基线或≤1级。Increase TARCEVA by 50 mg increments as tolerated for:
• Concomitant use with CYP3A4 inducers, such as rifampin, rifabutin, rifapentine, phenytoin, carbamazepine, phenobarbital, or St. John’s Wort. Increase doses by 50 mg increments at 2 week intervals to a maximum of 450 mg. Avoid concomitant use, if possible [see Drug Interactions (7)]. • Concurrent cigarette smoking. Increase by 50 mg increments at 2 week intervals to a maximum of 300 mg. Immediately reduce the dose of TARCEVA to the recommended dose (150 mg or 100 mg daily) upon cessation of smoking [see Drug Interactions (7) and Clinical Pharmacology (12.3)].Drugs Affecting Gastric pH
•如果可能的话,避免将Tarceva与质子泵抑制剂一起使用。剂量的分离可能不会消除相互作用,因为质子泵抑制剂会长时间影响上胃肠道的pH值。•如果需要用H 2受体拮抗剂(例如ranitidine)治疗,则必须在H 2受体拮抗剂给药后10小时进行TARCEVA,并且在下一次H 2受体拮抗剂之前至少2小时。•尽管尚未评估抗酸剂对erlotinib药代动力学的影响,但如果需要抗酸,抗酸剂量和TARCEVA剂量应分离几个小时。
登录到您的免费帐户


![Tarceva (Erlotinib Hydrochloride) Tablet [Genentech, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=57bccb29-1c47-4c64-ab6a-77960a91cc20&name=8c1ddd38-046e-46c6-9e57-cc6f0e0f4357-07.jpg)