FDA记录表明,这种药物目前没有召回。beplay sport中心钱包
您是医疗专业人员吗?
Trending Topics
Celexa回忆
获取警报召回时。
Questions & Answers
副作用和不良反应
警告临床恶化和自杀风险临床恶化和自杀风险
大抑郁症患者(MDD)(成人和小儿)可能会导致抑郁症和/或自杀念头和行为的出现(自杀性)或行为上的异常变化,无论他们是否正在服用抗抑郁药,这一点风险可能会持续到重大缓解为止。自杀是抑郁症和某些其他精神疾病的已知风险,这些疾病本身是自杀的最强预测指标。但是,长期以来一直担心的是,抗抑郁药在治疗的早期阶段可能会导致某些患者的抑郁症恶化和自杀性的出现。
Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided inTable 1.
| Age Range | 每1000名接受治疗的患者的自杀案件数量的药物 - 地位差异 |
| Increases Compared to Placebo |
|
| <18 | 14个其他案例 |
| 18-24 | 5 additional cases |
| 减少 Compared to Placebo |
|
| 25-64 | 1fewer case |
| >65 | 较少的案件 |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
所有接受抗抑郁药治疗的患者应适当监测任何适应症,并仔细观察到临床恶化,自杀性和行为不寻常的变化,尤其是在药物治疗过程的最初几个月中,或者在剂量变化的最初几个月中,要么增加,要么增加或减少。
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
应考虑改变治疗方案,包括可能停止药物,抑郁症持续恶化,或经历紧急自杀或可能是恶化抑郁症或自杀性的前体的患者,尤其是这些症状,尤其是这些症状,尤其在一开始,或不属于患者症状的一部分。
如果已经决定停止治疗ment, medication should be tapered, as rapidly as is feasible, but with recognition that abrupt discontinuation can be associated with certain symptoms (see PRECAUTIONS and DOSAGE AND ADMINISTRATION—Discontinuation of Treatment with Celexa, for a description of the risks of discontinuation of Celexa.
应警报用抗抑郁药治疗患者的家庭和护理人员因精神病和非精神病学的重大抑郁症或其他适应症的治疗,应注意监测患者的躁动出现,易怒,行为不寻常的变化以及上述其他症状的需求。,以及自杀的出现,并立即向医疗保健提供者报告此类症状。这种监控应包括家庭和看护人的每日观察。为了降低过量的风险,应将CELEXA的处方写成最少的片剂,以降低良好的患者管理。
筛查躁郁症患者:严重抑郁发作可能是最初的总统entation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that Celexa is not approved for use in treating bipolar depression.
与单胺氧化酶抑制剂相互作用的潜力在接受5-羟色胺再摄取抑制剂与单胺氧化酶抑制剂(MAOI)结合使用的患者中,有报道称严重的,有时是致命的反应,包括高温,刚性,肌昂尼斯,自主神经,自主不稳定,并可能快速波动的重要体征变化以及心理状况变化以及心理状况变化的快速波动这包括极端的搅动发展到ir妄和昏迷。这些反应在最近停止SSRI治疗并开始使用MAOI的患者中也有报道。某些情况表现出类似于神经蛋白质恶性肿瘤综合征的特征。此外,关于SSRI和MAOI联合使用影响的动物数据有限,表明这些药物可以协同起作用以升高血压并引起行为激发。因此,建议不应与MAOI结合使用Celexa,也不应在停用MAOI治疗后的14天内使用。同样,在开始MAOI之前停止Celexa后,至少应允许14天。
5-羟色胺综合征或神经蛋白质恶性综合征(NMS)样反应The development of a potentially life-threatening serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions have been reported with SNRIs and SSRIs alone, including Celexa treatment, but particularly with concomitant use of serotonergic drugs (including triptans) with drugs which impair metabolism of serotonin (including MAOIs), or with antipsychotics or other dopamine antagonists. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Serotonin syndrome, in its most severe form can resemble neuroleptic malignant syndrome, which includes hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation of vital signs, and mental status changes. Patients should be monitored for the emergence of serotonin syndrome or NMS-like signs and symptoms.
禁忌使用Celexa与毛伊斯的使用量用于治疗抑郁症。如果在临床上保证对CELEXA伴随治疗Celexa用5-羟色胺受体激动剂(Triptan)进行临床保证,则建议对患者进行仔细的观察,尤其是在治疗开始和剂量增加过程中。
The concomitant use of Celexa with serotonin precursors (such as tryptophan) is not recommended. Treatment with Celexa and any concomitant serotonergic or antidopaminergic agents, including antipsychotics, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
法律问题
目前尚无该药物可用的法律信息。
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
目前尚无该药物可用的制造商警告信息。
FDA Labeling Changes
目前,该药物尚无FDA标签更改。
Uses
Celexa (citalopram HBr) is indicated for the treatment of depression.
CELEXA在抑郁症治疗中的功效是在4-6周建立的,对门诊患者的对照试验,其诊断最与DSM-III和DSM-III和DSM-III-R类别相对应的主要抑郁症类别(请参阅临床药理学).
一个重大的抑郁发作(DSM-IV)意味着一个突出且相对持久的(几乎每天至少每天至少2周)抑郁或烦躁不安,通常会干扰日常功能,其中至少包括以下九种症状中的五个:失去对常规活动的兴趣,体重和/或食欲的显着变化,失眠或高血压,心理运动的躁动或迟缓,疲劳增加,内gui感或毫无价值的感觉,思维速度减慢或集中度降低,自杀尝试或自杀念头。
Celexa在住院的抑郁症患者中的抗抑郁作用尚未得到充分研究。
在两项安慰剂对照试验中,证明了Celexa在6至8周进行急性治疗后长达24周保持抗抑郁反应的功效(请参阅临床药理学). Nevertheless, the physician who elects to use Celexa for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
历史
There is currently no drug history available for this drug.
其他信息
CELEXA®(Citalopram HBR)是一种口服的选择性5-羟色胺再摄取抑制剂(SSRI),其化学结构与其他SSRI或三环,四环或其他可用抗抑郁药的化学结构无关。西妥丙酰亚普兰hbr是一种外围的双环邻苯二甲烷衍生物(±)-1-(3-二甲基氨基丙基)-1-(4-氟苯基)-1,3-二氢化苯二苯甲酰苯二苯甲酰苯二苯甲酰苯基-5-碳二硝基,HBR,HBR,HBR,具有以下结构公式:以下结构公式:
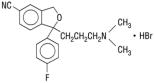
The molecular formula is C20H22BrFN2O及其分子量为405.35。
Citalopram HBr occurs as a fine, white to off-white powder. Citalopram HBr is sparingly soluble in water and soluble in ethanol.
Celexa (citalopram hydrobromide) is available as tablets or as an oral solution.
Celexa 10毫克片剂包膜缓释,椭圆形平板电脑containing citalopram HBr in strengths equivalent to 10 mg citalopram base. Celexa 20 mg and 40 mg tablets are film-coated, oval, scored tablets containing citalopram HBr in strengths equivalent to 20 mg or 40 mg citalopram base. The tablets also contain the following inactive ingredients: copolyvidone, corn starch, crosscarmellose sodium, glycerin, lactose monohydrate, magnesium stearate, hypromellose, microcrystalline cellulose, polyethylene glycol, and titanium dioxide. Iron oxides are used as coloring agents in the beige (10 mg) and pink (20 mg) tablets.
Celexa口服溶液中含有相当于2 mg/ml西妥位酰基碱基的西妥位HBR。它还包含以下不活跃成分:山梨糖醇,纯净水,丙烯乙二醇,甲基丙苯甲,天然薄荷味和丙酸糖蛋白酶。
Sources
Celexa制造商
-
医师Care,Inc。
![Celexa (Citalopram Hydrobromide) Tablet [Physicians Total Care, Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
celexa |医师Care,Inc。
![Celexa (Citalopram Hydrobromide) Tablet [Physicians Total Care, Inc.] Celexa (Citalopram Hydrobromide) Tablet [Physicians Total Care, Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Initial TreatmentCelexa (citalopram HBr) should be administered at an initial dose of 20 mg once daily, generally with an increase to a dose of 40 mg/day. Dose increases should usually occur in increments of 20 mg at intervals of no less than one week. Although certain patients may require a dose of 60 mg/day, the only study pertinent to dose response for effectiveness did not demonstrate an advantage for the 60 mg/day dose over the 40 mg/day dose; doses above 40 mg are therefore not ordinarily recommended.
Celexa should be administered once daily, in the morning or evening, with or without food.
Special Populations对于大多数老年患者和肝损伤患者的建议剂量为20 mg/天,滴定为40 mg/天,仅针对无反应的患者。
No dosage adjustment is necessary for patients with mild or moderate renal impairment. Celexa should be used with caution in patients with severe renal impairment.
Treatment of Pregnant Women During the Third Trimester在第三学期晚些时候,暴露于Celexa和其他SSRI或SNRI的新生儿已经出现了需要长时间住院,呼吸支持和管喂养的并发症(请参阅预防措施)。在三个月治疗Celexa的孕妇时,医生应仔细考虑治疗的潜在风险和益处。医师可以考虑在三个月逐渐变细的Celexa。
维护处理It is generally agreed that acute episodes of depression require several months or longer of sustained pharmacologic therapy. Systematic evaluation of Celexa in two studies has shown that its antidepressant efficacy is maintained for periods of up to 24 weeks following 6 or 8 weeks of initial treatment (32 weeks total). In one study, patients were assigned randomly to placebo or to the same dose of Celexa (20-60 mg/day) during maintenance treatment as they had received during the acute stabilization phase, while in the other study, patients were assigned randomly to continuation of Celexa 20 or 40 mg/day, or placebo, for maintenance treatment. In the latter study, the rates of relapse to depression were similar for the two dose groups (see Clinical Trials under CLINICAL PHARMACOLOGY). Based on these limited data, it is not known whether the dose of citalopram needed to maintain euthymia is identical to the dose needed to induce remission. If adverse reactions are bothersome, a decrease in dose to 20 mg/day can be considered.
用Celexa治疗中断Symptoms associated with discontinuation of Celexa and other SSRIs and SNRIs have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
切换患者往返单胺氧化酶抑制剂At least 14 days should elapse between discontinuation of an MAOI and initiation of Celexa therapy. Similarly, at least 14 days should be allowed after stopping Celexa before starting an MAOI (see CONTRAINDICATIONS and WARNINGS).
-
伊利湖医疗和外科供应DBA质量护理产品有限责任公司
![Celexa(Citalopram Hydrobromide)片剂,膜涂层[伊利湖医疗和外科供应DBA质量护理产品有限责任公司]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
celexa |伊利湖医疗和外科供应DBA质量护理产品有限责任公司
![Celexa (Citalopram Hydrobromide) Tablet, Film Coated [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc ] Celexa(Citalopram Hydrobromide)片剂,膜涂层[伊利湖医疗和外科供应DBA质量护理产品有限责任公司]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Celexa should be administered once daily, in the morning or evening, with or without food.
Initial TreatmentCelexa(西妥位HBR)应以每天20 mg的初始剂量施用,每天增加40 mg/天的最大剂量。剂量增加通常应以20毫克的增量,每隔不少于一周。由于QT延长的风险,不建议服用40毫克/天以上的剂量。此外,与剂量反应有关的唯一研究并未证明40 mg/天剂量在40 mg/天剂量上的优势。
Special Populations对于大多数老年患者和肝损伤患者的建议剂量为20 mg/天,滴定为40 mg/天,仅针对无反应的患者。20毫克/天是CYP2C19差代谢者或服用西米替丁或其他CYP2C19抑制剂的患者的最大建议剂量。
No dosage adjustment is necessary for patients with mild or moderate renal impairment. Celexa should be used with caution in patients with severe renal impairment.
Treatment of Pregnant Women During the Third Trimester在第三学期晚些时候,暴露于Celexa和其他SSRI或SNRI的新生儿已经出现了需要长时间住院,呼吸支持和管喂养的并发症(请参阅预防措施)。在三个月治疗Celexa的孕妇时,医生应仔细考虑治疗的潜在风险和益处。医师可以考虑在三个月逐渐变细的Celexa。
维护处理It is generally agreed that acute episodes of depression require several months or longer of sustained pharmacologic therapy. Systematic evaluation of Celexa in two studies has shown that its antidepressant efficacy is maintained for periods of up to 24 weeks following 6 or 8 weeks of initial treatment (32 weeks total). In one study, patients were assigned randomly to placebo or to the same dose of Celexa (20-60 mg/day) during maintenance treatment as they had received during the acute stabilization phase, while in the other study, patients were assigned randomly to continuation of Celexa 20 or 40 mg/day, or placebo, for maintenance treatment. In the latter study, the rates of relapse to depression were similar for the two dose groups (see Clinical Trials under CLINICAL PHARMACOLOGY). Based on these limited data, it is not known whether the dose of citalopram needed to maintain euthymia is identical to the dose needed to induce remission. If adverse reactions are bothersome, a decrease in dose to 20 mg/day can be considered.
用Celexa治疗中断Symptoms associated with discontinuation of Celexa and other SSRIs and SNRIs have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
切换患者往返单胺氧化酶抑制剂At least 14 days should elapse between discontinuation of an MAOI and initiation of Celexa therapy. Similarly, at least 14 days should be allowed after stopping Celexa before starting an MAOI (see CONTRAINDICATIONS and WARNINGS).
-
森林实验室公司
![Celexa (Citalopram Hydrobromide) Tablet, Film Coated [Forest Laboratories, Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
celexa |森林实验室公司
![Celexa (Citalopram Hydrobromide) Tablet, Film Coated [Forest Laboratories, Inc.] Celexa (Citalopram Hydrobromide) Tablet, Film Coated [Forest Laboratories, Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Celexa should be administered once daily, in the morning or evening, with or without food.
Initial TreatmentCELEXA(西妥位HBR)应以每天20 mg的初始剂量为20 mg,并以不少于一周的时间间隔增加40 mg/天的最大剂量。由于QT延长的风险,不建议服用40毫克/天以上的剂量。此外,与剂量反应有关的唯一研究并未证明40 mg/天剂量在40 mg/天剂量上的优势。
Special Populations20mg/day is the maximum recommended dose for patients who are greater than 60 years of age, patients with hepatic impairment, and for CYP2C19 poor metabolizers or those patients taking cimetidine or another CYP2C19 inhibitor. (see WARNINGS)
No dosage adjustment is necessary for patients with mild or moderate renal impairment. Celexa should be used with caution in patients with severe renal impairment.
Treatment of Pregnant Women During the Third Trimester在第三学期晚些时候,暴露于Celexa和其他SSRI或SNRI的新生儿已经出现了需要长时间住院,呼吸支持和管喂养的并发症(请参阅预防措施)。在三个月治疗Celexa的孕妇时,医生应仔细考虑治疗的潜在风险和益处。
维护处理It is generally agreed that acute episodes of depression require several months or longer of sustained pharmacologic therapy. Systematic evaluation of Celexa in two studies has shown that its antidepressant efficacy is maintained for periods of up to 24 weeks following 6 or 8 weeks of initial treatment (32 weeks total). In one study, patients were assigned randomly to placebo or to the same dose of Celexa (20-60 mg/day) during maintenance treatment as they had received during the acute stabilization phase, while in the other study, patients were assigned randomly to continuation of Celexa 20 or 40 mg/day, or placebo, for maintenance treatment. In the latter study, the rates of relapse to depression were similar for the two dose groups (see Clinical Trials under CLINICAL PHARMACOLOGY). Based on these limited data, it is not known whether the dose of citalopram needed to maintain euthymia is identical to the dose needed to induce remission. If adverse reactions are bothersome, a decrease in dose to 20 mg/day can be considered.
用Celexa治疗中断Symptoms associated with discontinuation of Celexa and other SSRIs and SNRIs have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders至少14天应该在旨在治疗精神疾病的MAOI的停用与使用Celexa治疗的情况下进行。相反,在开始使用旨在治疗精神疾病的MAOI之前停止Celexa后,至少应允许14天(请参阅禁忌症)。
将Celexa与其他MAOI(例如Linezolid或亚甲基蓝)一起使用Do not start Celexa in a patient who is being treated with linezolid or intravenous methylene blue because there is an increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered (see CONTRAINDICATIONS).
In some cases, a patient already receiving Celexa therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, Celexa should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with Celexa may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (see WARNINGS).
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with Celexa is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use (see WARNINGS).
Login To Your Free Account


![Celexa(Citalopram Hydrobromide)片剂,膜涂层[伊利湖医疗和外科供应DBA质量护理产品有限责任公司]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=484db0b4-f7dc-4fd6-b279-00104e92bd1b&name=celexa40mgforest.jpg)
![Celexa (Citalopram Hydrobromide) Tablet, Film Coated [Forest Laboratories, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=4259d9b1-de34-43a4-85a8-41dd214e9177&name=cel00-0019-02.jpg)