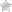WARNING - FDA records indicate that this drug has been recalled.
| 产品描述: | Cefoxitin for Injection and Dextrose Injection, 1 g in Duplex, 50 mL Container, Catalog No. 3123-11, For IV Use Only, Single Use, Sterile, Rx Only, B Braun Medical Inc, Irvine CA 92614-5895, NDC 0264-3123-11 |
|---|---|
| 地位: | Ongoing |
| City: | Irvine |
| state: | CA |
| 国家: | US |
| 自愿/要求: | Voluntary: Firm Initiated |
| Initial Firm Notification: | Letter |
| Distribution Pattern: | nationwide, Puerto Rico and Spain |
| Classification: | Class I |
| 产品数量: | 22,584 units |
| reason For Recall: | Presence of Particulate Matter: B. Braun Medical Inc. is recalling several injectable products due to visible particulate matter found in reserve sample units. |
| 记起Initiation Date: | 20131121 |
| 报告日期: | 20140507 |
Are you a medical professional?
Trending Topics
Cefoxitin And Dextrose Recall
Get an alertwhen a recall is issued.
Questions & Answers
side Effects & Adverse Reactions
BEFORE THERAPY WITH CEFOXITIN FOR INJECTION AND DEXTROSE INJECTION IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFOXITIN, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. THIS PRODUCT SHOULD BE GIVEN WITH CAUTION TO PENICILLIN-SENSITIVE PATIENTS. ANTIBIOTICS SHOULD BE ADMINISTERED WITH CAUTION TO ANY PATIENT WHO HAS DEMONSTRATED SOME FORM OF ALLERGY, PARTICULARLY TO DRUGS. IF AN ALLERGIC REACTION TO CEFOXITIN FOR INJECTION AND DEXTROSE INJECTION OCCURS, DISCONTINUE THE DRUG. SERIOUS HYPERSENSITIVITY REACTIONS MAY REQUIRE EPINEPHRINE AND OTHER EMERGENCY MEASURES.
艰难梭菌据报道,相关的腹泻(CDAD)使用了几乎所有抗菌剂,包括用于注射和右旋糖的头孢辛蛋白,并且可能范围从轻度腹泻到致命结肠炎。用抗菌剂的治疗改变了结肠的正常菌群,导致过度生长艰难梭菌。
艰难梭菌produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of艰难梭菌导致发病率和死亡率提高,因为这些感染可能是对抗菌治疗的难治性,并且可能需要结肠切除术。在使用抗生素后,所有患有腹泻的患者必须考虑CDAD。由于据报道CDAD发生在抗菌剂后的两个月内,因此需要仔细的病史。
如果怀疑或确认了CDAD,则不针对持续的抗生素使用艰难梭菌may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of艰难梭菌, and surgical evaluation should be instituted as clinically indicated.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
目前尚无该药物可用的制造商警告信息。
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefoxitin for Injection and Dextrose Injection and other antibacterial drugs, Cefoxitin for Injection and Dextrose Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
用于注射和右旋糖注射的头孢辛素用于治疗以下疾病中指定的微生物易感性菌株引起的严重感染。
(1) Lower respiratory tract infections,including pneumonia and lung abscess, caused bystreptococcus pneumoniae, other streptococci (excluding enterococci, e.g.,Enterococcus faecalis[formerlystreptococcus faecalis]),,staphylococcus aureus(including penicillinase-producing strains),Escherichia coli, Klebsiellaspecies,嗜血杆菌流感, andBacteroidesspecies.
(2)尿路感染由Escherichia coli, Klebsiellaspecies,Proteus mirabilis,Morganella Morganii,Proteus dulgarisandProvidencia物种(包括P. rettgeri).
(3) Intra-abdominal infections,including peritonitis and intra-abdominal abscess, caused byEscherichia coli, Klebsiellaspecies,Bacteroides包括细菌性脆弱, and梭状芽胞杆菌species.
(4) Gynecological infections,包括子宫内膜炎,骨盆蜂窝织炎和骨盆炎性疾病Escherichia coli, Neisseria gonorrhoeae(including penicillinase-producing strains),Bacteroides包括B. Fragilis,梭状芽胞杆菌species,Peptococcus niger, Peptostreptococcusspecies, and链球菌agalactiae。Cefoxitin, like cephalosporins, has no activity against衣原体沙眼。Therefore, when cefoxitin is used in the treatment of patients with pelvic inflammatory disease andC.气管is one of the suspected pathogens, appropriate anti-chlamydial coverage should be added.
(5) Septicemia由streptococcus pneumoniae, Staphylococcus aureus(including penicillinase-producing strains),Escherichia coli, Klebsiellaspecies, andBacteroides包括B. fragilis。
(6) Bone and joint infections由staphylococcus aureus(including penicillinase-producing strains).
(7) Skin and skin structure infections由staphylococcus aureus(including penicillinase-producing strains),staphylococcus epidermidis, Streptococcus pyogenesand other streptococci (excluding enterococci, e.g.,Enterococcus faecalis[formerlystreptococcus faecalis]),大肠杆菌,Proteus mirabilis,Klebsiellaspecies,Bacteroides包括B. Fragilis,梭状芽胞杆菌species,Peptococcus niger, andPeptostreptococcusspecies.
Appropriate culture and susceptibility studies should be performed to determine the susceptibility of the causative organisms to cefoxitin. Therapy may be started while awaiting the results of these studies.
在随机比较研究中,头孢辛蛋白和头孢霉素在由革兰氏阳性球菌和革兰氏阴性杆上引起的感染的治疗中相当安全且有效。在存在细菌β-内酰胺酶(青霉素酶和头孢菌素酶)的情况下,头孢辛蛋白具有高度的稳定性。
Many infections caused by aerobic and anaerobic Gram-negative bacteria resistant to some cephalosporins respond to cefoxitin. Similarly, many infections caused by aerobic and anaerobic bacteria resistant to some penicillin antibiotics (ampicillin, carbenicillin, penicillin G) respond to treatment with cefoxitin. Many infections caused by mixtures of susceptible aerobic and anaerobic bacteria respond to treatment with cefoxitin.
Cefoxitin is indicated for the prophylaxis of infection in patients undergoing uncontaminated gastrointestinal surgery, vaginal hysterectomy, abdominal hysterectomy, or cesarean section.
If there are signs of infection, specimens for culture should be obtained for identification of the causative organism so that appropriate treatment may be instituted.
历史
There is currently no drug history available for this drug.
Other Information
The drug chamber is filled with cefoxitin sodium USP, a semi-synthetic, broad-spectrum cepha antibiotic sealed under nitrogen for intravenous administration. It is derived from cephamycin C, which is produced by链霉菌。Its chemical name is sodium (6r,7s)-3-(羟甲基)-7-甲氧基-8-OXO-7- [2-(2-硫烯基)乙酰胺] -5- thia-1-1- azabicyclo [4.2.0] oct-2-2-end-2-ene-2-烯键羧酸盐氨基甲酸酯(酯)。经验公式是C16H16n3naO7s2,分子量449.44。方法,al formula is:
Cefoxitin sodium contains approximately 53.8 mg (2.3 mEq) of sodium per gram of cefoxitin activity.
Cefoxitin for Injection and Dextrose Injection is supplied as a sterile, nonpyrogenic, single use packaged combination of cefoxitin (filled using Cefoxitin Sodium USP) and Dextrose Injection (diluent). After reconstitution, each 50 mL contains cefoxitin sodium equivalent to either 1 gram or 2 grams cefoxitin. The diluent chamber contains Dextrose Injection. The concentration of Dextrose Hydrous USP in Water for Injection USP has been adjusted to render the reconstituted drug product iso-osmotic. Dextrose Hydrous USP has been added to adjust the osmolality to approximately 290 mOsmol/kg (approximately 2 g (4% w/v) and 1.1 g (2.2% w/v) to the 1 g and 2 g doses, respectively). Dextrose Injection is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents.
葡萄糖液压USP具有以下结构(分子)公式:
The molecular weight of Dextrose Hydrous USP is 198.17.
After removing the peelable foil strip, activating the seals, and thoroughly mixing, the reconstituted drug product is intended for single intravenous use. When reconstituted according to instructions in the product labeling, the approximate osmolality of the reconstituted solution of Cefoxitin for Injection and Dextrose Injection is approximately 290 mOsmol/kg. After reconstitution, the pH is approximately 6.5. Solutions of Cefoxitin for Injection and Dextrose Injection range from colorless to light amber.
not made with natural rubber latex, PVC or Di(2-ethylhexyl)phthalate (DEHP).
The DUPLEX® dual chamber container is made from a specially formulated material. The product (diluent and drug) contact layer is a mixture of thermoplastic rubber and a polypropylene ethylene copolymer that contains no plasticizers. The safety of the container system is supported by USP biological evaluation procedures.
sources
Cefoxitin And Dextrose Manufacturers
-
B. Braun Medical Inc.
![Cefoxitin And Dextrose (Cefoxitin Sodium) Injection, Solution [B. Braun Medical Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
头孢辛蛋白和葡萄糖|B. Braun Medical Inc.
![Cefoxitin And Dextrose (Cefoxitin Sodium) Injection, Solution [B. Braun Medical Inc.] Cefoxitin And Dextrose (Cefoxitin Sodium) Injection, Solution [B. Braun Medical Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Cefoxitin for Injection and Dextrose Injection in the DUPLEX® Container is intended for intravenous use only.
TREATMENT Adults通常的成人剂量范围为每六到八个小时1克至2克。剂量应取决于病因的敏感性,感染的严重程度和患者的状况(剂量指南请参见表3)。beplay体验
If C. trachomatis is a suspected pathogen, appropriate anti-chlamydial coverage should be added, because cefoxitin sodium has no activity against this organism.
Cefoxitin for Injection and Dextrose Injection may be used in patients with reduced renal function with the following dosage adjustments:
In adults with renal insufficiency, an initial loading dose of 1 gram to 2 grams may be given. After a loading dose, the recommendations for maintenance dosage (Table 4) may be used as a guide.
When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to convert this value into creatinine clearance. The serum creatinine should represent a steady state of renal function.
男性:体重(kg)×(140-年龄)72×血清肌酐(mg/100 mL)女性:0.85×高于值In patients undergoing hemodialysis, the loading dose of 1 to 2 grams should be given after each hemodialysis, and the maintenance dose should be given as indicated in Table 4.
Antibiotic therapy for group A beta-hemolytic streptococcal infections should be maintained for at least 10 days to guard against the risk of rheumatic fever or glomerulonephritis. In staphylococcal and other infections involving a collection of pus, surgical drainage should be carried out where indicated.
小儿患者The recommended dosage in pediatric patients three months of age and older is 80 to 160 mg/kg of body weight per day divided into four to six equal doses. The higher dosages should be used for more severe or serious infections. The total daily dosage should not exceed 12 grams.
At this time no recommendation is made for pediatric patients from birth to three months of age (see PRECAUTIONS).
在儿科患者肾功能不全,the dosage and frequency of dosage should be modified consistent with the recommendations for adults (see Table 4).
PREVENTIONEffective prophylactic use depends on the time of administration. Cefoxitin for Injection and Dextrose Injection usually should be given one-half to one hour before the operation, which is sufficient time to achieve effective levels in the wound during the procedure. Prophylactic administration should usually be stopped within 24 hours since continuing administration of any antibiotic increases the possibility of adverse reactions but, in the majority of surgical procedures, does not reduce the incidence of subsequent infection.
在未被污染的预防性使用gastrointestinal surgery, vaginal hysterectomy, or abdominal hysterectomy, the following doses are recommended:
Adults2grams administered intravenously just prior to surgery (approximately one-half to one hour before the initial incision) followed by 2 grams every 6 hours after the first dose for no more than 24 hours.
小儿患者(3个月以上)30to 40 mg/kg doses may be given at the times designated above.
Cesarean section patients对于接受剖宫产的患者,脐带夹紧后立即静脉内服用2克剂量,或者在静脉内固定后,由2克固定固定后,将脐带夹紧,然后夹紧2克4和8小时,然后静脉注射2克4和8小时建议初始剂量后。(请参阅临床研究。)
Table 3 - Guidelines for Dosage of Cefoxitin for Injection Type of Infection Daily Dosage Frequency and Route * Including patients in whom bacteremia is absent or unlikely. Uncomplicated forms*
of infections such as
pneumonia, urinary
tract infection, cutaneous
infection 3–4 grams 1 gram every 6–8 hours IV Moderately severe or
严重感染6-8克每4小时1克
or
2grams every 6–8 hours IV Infections commonly
needing antibiotics in
higher dosage (e.g.,
gas gangrene) 12 grams 2 grams every 4 hours
or
每6个小时3克iv表4-头孢辛蛋白的维持剂量用于肾功能降低肾功能肌酐清除率降低的成人
(mL/min) Dose
(grams) Frequency Mild impairment 50–30 1–2 every 8–12 hours Moderate impairment 29–10 1–2 every 12–24 hours Severe impairment 9–5 0.5–1 every 12–24 hours Essentially no function <5 0.5–1 every 24–48 hours ADMINISTRATIONThe intravenous route is preferable for patients with bacteremia, bacterial septicemia, or other severe or life-threatening infections, or for patients who may be poor risks because of lowered resistance resulting from such debilitating conditions as malnutrition, trauma, surgery, diabetes, heart failure, or malignancy, particularly if shock is present or impending.
Cefoxitin for Injection and Dextrose Injection may be administered through the tubing system by which the patient may be receiving other intravenous solutions. However, during infusion of the solution containing Cefoxitin for Injection and Dextrose Injection, it is advisable to temporarily discontinue administration of any other solutions at the same site.
头孢辛蛋白用于注射和葡萄糖注射的溶液,例如大多数β-内酰胺抗生素的溶液,不应将其添加到氨基糖苷溶液(例如,硫酸庆大霉素,硫酸甲状腺霉素,硫酸氨基氨基酸酯,硫酸氨基胺)中,因为潜在的相互作用是潜在的相互作用。然而,可以分别对同一患者分别给予注射和葡萄糖注射和氨基糖苷的头孢辛蛋白。
CAUTION: Do not use plastic containers in series connections. Such use would result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete.
nOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Duplex®药物输送系统用于避免无意激活的用途,Duplex®容器应保持在折叠位置,直到打算激活为止。Patient Labeling and Drug Powder/Diluent Inspection
Apply patient-specific label on foil side of container. USE CARE to avoid activation. Do not cover any portion of foil strip with patient label. Unlatch side tab and unfold Duplex® Container. (See Diagram 1.)
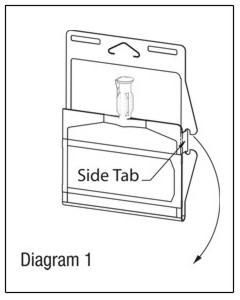
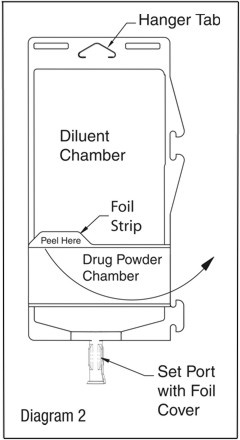
Visually inspect diluent chamber for particulate matter. Use only if container and seals are intact. To inspect the drug powder for foreign matter or discoloration, peel foil strip from drug chamber. (See Diagram 2.)
Protect from light after removal of foil strip.note: If foil strip is removed, product must be used within 7 days, but not beyond the labeled expiration date.
The product should be re-folded and the side tab latched until ready to activate.reconstitution (Activation)
不要使用后直接由制冷存储, allow the product to equilibrate to room temperature before patient use. Unfold the DUPLEX® Container and point the set port in a downward direction. Starting at the hanger tab end, fold the DUPLEX® Container just below the diluent meniscus trapping all air above the fold. To activate, squeeze the folded diluent chamber until the seal between the diluent and powder opens, releasing diluent into the drug powder chamber. (See Diagram 3.)
搅拌液 - 粉混合物,直到药粉完全溶解为止。注意:重建后(激活),如果在室温下存储,则必须在12小时内或在7天内存储在制冷中。
Administration
Visually inspect the reconstituted solution for particulate matter. Point the set port in a downwards direction. Starting at the hanger tab end, fold the DUPLEX® Container just below the solution meniscus trapping all air above the fold. Squeeze the folded DUPLEX® Container until the seal between reconstituted drug solution and set port opens, releasing liquid to set port. (See Diagram 4.)
在附加IV集之前,请牢固地挤压容器,检查是否有微小的泄漏。如果发现泄漏,则丢弃容器和解决方案,因为无菌性可能会受到损害。使用无菌技术,将箔纸从设定的端口覆盖并连接无菌给药组。(请参阅图5。)
请参阅行政集合随附的说明。Precautions
As with other cephalosporins, reconstituted Cefoxitin for Injection and Dextrose Injection tends to darken depending on storage conditions, within the stated recommendations. However, product potency is not adversely affected. Use only if prepared solution is clear and free from particulate matter. Do not use in series connection. Do not introduce additives into the DUPLEX® Container. Do not freeze.
Login To Your Free Account