警告-FDA记录表明,该药物已被召回。
| Product Description: | Cefazolin for Injection USP and Dextrose Injection USP, 2 g in Duplex, 50 mL Container, Catalog Number 3105-11, Rx Only, B Braun Medical Inc., Irvine CA 92614, NDC 0264-3105-11 |
|---|---|
| Status: | 正在进行 |
| 城市: | Irvine |
| 状态: | CA |
| Country: | US |
| Voluntary/Mandated: | 自愿:公司发起的公司 |
| Initial Firm Notification: | Letter |
| 分配模式: | 全国,波多黎各和西班牙 |
| 分类: | Class I |
| Product Quantity: | 42,576 units |
| Reason For Recall: | 颗粒物的存在:B。Braun Medical Inc.正在召回几种可在储备样品单元中发现的可见颗粒物而导致的注射产品。 |
| Recall Initiation Date: | 20131121 |
| Report Date: | 20140507 |
Are you a medical professional?
热门话题
头孢唑素钠溶液Recall
Get an alertwhen a recall is issued.
问题和答案
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
目前还没有生产informa的警告tion available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
用途
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefazolin for Injection USP and Dextrose Injection USP and other antibacterial drugs, Cefazolin for Injection USP and Dextrose Injection USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
在易感细菌引起的情况下,指示用于注射USP和右旋糖注射USP的头孢唑林USP治疗以下感染。
Respiratory tract infections due toStreptococcus pneumoniae, Staphylococcus aureus和Streptococcus pyogenes.
Injectable benzathine penicillin is considered the drug of choice in treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever.
Cefazolin is effective in the eradication of streptococci from the nasopharynx; however, data establishing the efficacy of cefazolin in the subsequent prevention of rheumatic fever are not available.
Urinary tract infections due toEscherichia coli,和Proteus mirabilis.
Skin and skin structure infections due toS. aureus,S. pyogenes, andStreptococcus agalactiae.
Biliary infections due toE. coli, various isolates of streptococci,P. mirabilis,和S. aureus.
骨和关节感染S. aureus.
生殖器感染E. coli,和P. mirabilis.
Septicemia due toS. Pneumoniae,S。金黄色,P. mirabilis,和E. coli.
Endocarditis due toS. aureus和S. pyogenes.
术前,术中和术后预防性服用头孢唑素可能会减少某些术后感染的发生率70年,患有急性胆囊炎,阻塞性黄疸或普通管道胆结石)。
头孢唑啉的围手术期使用也可以滚开ective in surgical patients in whom infection at the operative site would present a serious risk (e.g., during open-heart surgery and prosthetic arthroplasty).
If there are signs of infection, specimens for cultures should be obtained for the identification of the causative organism so that appropriate therapy may be instituted.
History
There is currently no drug history available for this drug.
Other Information
Cefazolin for Injection USP and Dextrose Injection USP is a sterile, nonpyrogenic, single use, packaged combination of Cefazolin Sodium USP (lyophilized) and sterile iso-osmotic diluent in the DUPLEX® sterile container. The DUPLEX® Container is a flexible dual chamber container.
After reconstitution the approximate osmolality for Cefazolin for Injection USP and Dextrose Injection USP is 290 mOsmol/kg.
The drug chamber is filled with sterile lyophilized Cefazolin Sodium USP, a semi-synthetic cephalosporin and has the following IUPAC nomenclature: Sodium (6R,7R)-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[2-(1H-tetrazol-1-yl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate.
Cefazolin Sodium USP has the following structural formula:
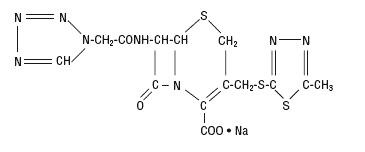
The sodium content is 48 mg/g of cefazolin sodium.
The diluent chamber contains Dextrose Injection USP, an iso-osmotic diluent using Hydrous Dextrose USP in Water for Injection USP. Dextrose Injection USP is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents.
Hydrous Dextrose USP has the following structural (molecular) formula:
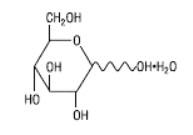
The molecular weight of Hydrous Dextrose USP is 198.17
Cefazolin Sodium USP is supplied as a lyophilized form equivalent to either 1 g or 2 g of cefazolin. Dextrose hydrous USP has been added to the diluent to adjust osmolality (approximately 2 g [4.0% w/v] and 1.5 g [3.0% w/v] for the 1 g and 2 g dosages, respectively).
After removing the peelable foil strip, activating the seals, and thoroughly mixing, the reconstituted drug product is intended for single intravenous use.
The DUPLEX® Container is not manufactured with Latex, PVC or DEHP.
The DUPLEX® dual chamber container is made from a specially formulated material. The product (diluent and drug) contact layer is a mixture of thermoplastic rubber and a polypropylene ethylene copolymer that contains no plasticizers. The safety of the container system is supported by USP biological evaluation procedures.
Sources
头孢唑素钠溶液Manufacturers
-
B. Braun Medical Inc.
![头孢唑素钠溶液[B. Braun Medical Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
头孢唑素钠溶液| Heritage Pharmaceuticals Inc.
![Cefazolin Sodium Solution [B. Braun Medical Inc.] 头孢唑素钠溶液[B. Braun Medical Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Infusion-related events are related to both the concentration and the rate of administration of vancomycin. Concentrations of no more than 5 mg/mL and rates of no more than 10 mg/min are recommended in adults (see also age-specific recommendations). In selected patients in need of fluid restriction, a concentration up to 10 mg/mL may be used; use of such higher concentrations may increase the risk of infusion related events. An infusion rate of 10 mg/min or less is associated with fewer infusion-related events (see ADVERSE REACTIONS). Infusion-related events may occur, however, at any rate or concentration.
肾功能正常的患者
Adults
The usual daily intravenous dose is 2 g divided either as 500 mg every 6 hours or 1 g every 12 hours.
Each dose should be administered at no more than 10 mg/min, or over a period of at least 60 minutes, whichever is longer. Other patient factors, such as age or obesity, may call for modification of the usual intravenous daily dose.
Pediatric Patients
The usual intravenous dosage of vancomycin is 10 mg/kg per dose given every 6 hours. Each dose should be administered over a period of at least 60 minutes.Close monitoring of serum concentrations of vancomycin may be warranted in these patients.
Neonates
在1个月大的儿科患者中,每日静脉内剂量可能较低。在新生儿中,建议初始剂量为15 mg/kg,然后在生命的第1周,每12小时每12小时,每12小时,此后每8小时每8小时,直到1个月。每剂应在60分钟内给药。在过早的婴儿中,万古霉素的清除率随着概念后年龄的降低而降低。因此,在早产婴儿中可能需要更长的给药间隔。在这些患者中建议密切监测万古霉素的血清浓度。
肾功能受损和老年患者的患者
肾功能受损的患者必须进行剂量调整。在早产儿和老年人中,由于肾功能降低,可能需要比预期的剂量降低更多。万古霉素血清浓度的测量可能有助于优化治疗,尤其是在肾功能变化的严重患者中。可以通过使用微生物学测定,放射免疫测定,荧光极化免疫测定,荧光免疫测定或高压液相色谱来确定万古霉素血清浓度。
If creatinine clearance can be measured or estimated accurately, the dosage for most patients with renal impairment can be calculated using the following table. The dosage of vancomycin hydrochloride for injection per day in mg is about 15 times the glomerular filtration rate in mL/min (see following table).
肾功能受损患者万古霉素的剂量表
(Adapted from Moellering et al.4)
Creatinine Clearance
Vancomycin Dose
mL/min
mg/24 h
100
1,545
90
1,390
80
1,235
70
1,080
60
925
50
770
40
620
30
465
20
310
10
155
The initial dose should be no less than 15 mg/kg, even in patients with mild to moderate renal insufficiency.
The table is not valid for functionally anephric patients. For such patients, an initial dose of 15 mg/kg of body weight should be given to achieve prompt therapeutic serum concentrations.
The dose required to maintain stable concentrations is 1.9 mg/kg/24 hr. In patients with marked renal impairment, it may be more convenient to give maintenance doses of 250 to 1,000 mg once every several days rather than administering the drug on a daily basis. In anuria, a dose of 1,000 mg every 7 to 10 days has been recommended.
当仅知道血清肌酐时,可以使用以下配方(基于性别,体重和年龄)来计算肌酐清除率。计算出的肌酐清除率(mL/min)仅是估计值。肌酐清除率应及时测量。
男性:[体重(kg)X(140 - 年龄年龄)]
72 x serum creatinine concentration (mg/dL)
Women: 0.85 x above value
The serum creatinine must represent a steady state of renal function. Otherwise the estimated value for creatinine clearance is not valid. Such a calculated clearance is an overestimate of actual clearance in patients with conditions: (1) characterized by decreasing renal function, such as shock, severe heart failure or oliguria; (2) in which a normal relationship between muscle mass and total body weight is not present, such as in obese patients or those with liver disease, edema or ascites; and (3) accompanied by debilitation, malnutrition or inactivity.
The safety and efficacy of vancomycin administration by the intrathecal (intralumbar or intraventricular) routes have not been established. Intermittent infusion is the recommended method of administration.
Compatibility with Other Drugs and IV Fluids
以下稀释剂在物理和化学上兼容(盐酸4 g/L万古霉素):
5% Dextrose Injection, USP
5% Dextrose Injection and 0.9% Sodium Chloride Injection, USP
Lactated Ringer's Injection, USP
5% Dextrose and Lactated Ringer's Injection
Normosol®-M and 5% Dextrose
0.9% Sodium Chloride Injection, USP
Isolyte® E
Good professional practice suggests that compounded admixtures should be administered as soon after preparation as is feasible.
Vancomycin solution has a low pH and may cause physical instability of other compounds.
Mixtures of solutions of vancomycin and beta-lactam antibiotics have been shown to be physically incompatible. The likelihood of precipitation increases with higher concentrations of vancomycin. It is recommended to adequately flush the intravenous lines between the administration of these antibiotics. It is also recommended to dilute solutions of vancomycin to 5 mg/mL or less.
Although intravitreal injection is not an approved route of administration for vancomycin, precipitation has been reported after intravitreal injection of vancomycin and ceftazidime for endophthalmitis using different syringes and needles. The precipitates dissolved gradually, with complete clearing of the vitreous cavity over two months and with improvement of visual acuity.
PREPARATION AND STABILITY
Directions for Proper Use of a Pharmacy Bulk Package
DIRECTIONS FOR PROPER USE OF PHARMACY BULK PACKAGE
不是直接注入。药房散装包装is for use in the Pharmacy Admixture Service only in a suitable work area such as a laminar flow hood. Using aseptic technique, the closure may be penetrated only one time after reconstitution using a suitable sterile transfer device or dispensing set, which allows measured dispensing of the contents. Use of a syringe and needle is not recommended as it may cause leakage. After entry use entire contents of the Pharmacy Bulk Package bottle promptly. The entire contents of the Pharmacy Bulk Package bottle should be dispensed within 4 hours after initial entry. A maximum time of 4 hours from the initial entry may be allowed to complete fluid aliquoting/transferring operations. Discard the container no later than 4 hours after initial closure puncture. This time limit should begin with the introduction of solvent or diluent into the Pharmacy Bulk Package bottle.
Preparation and Stability
10 g Pharmacy Bulk Package bottle
At the time of use, reconstitute by adding 95 mL of Sterile Water for Injection, USP to the 10 g bottle of dry, sterile vancomycin powder. The resultant solution will contain vancomycin equivalent to 500 mg/5 mL (1 g/10 mL). AFTER RECONSTITUTION, FURTHER DILUTION IS REQUIRED.
必须在至少100 mL合适的输注溶液中进一步稀释万古霉素(500 mg/5 mL)的重构溶液。对于1克(10 mL)的剂量,必须使用至少200毫升溶液。以这种方式稀释的所需剂量应在至少60分钟内通过间歇性IV输注来给药。
Parenteral drug products should be visually inspected for particulate matter and discoloration prior to administration, whenever solution and container permit.
For Oral Administration
Oral vancomycin is used in treating antibiotic -associated pseudomembranous colitis caused by C. difficile and for staphylococcal enterocolitis. Vancomycin is not effective by the oral route for other types of infections. The usual adult total daily dosage is 500 mg to 2 g given in 3 or 4 divided doses for 7 to 10 days. The total daily dose in children is 40 mg/kg of body weight in 3 or 4 divided doses for 7 to 10 days. The total daily dosage should not exceed 2 g. The appropriate dose may be diluted in 1 oz of water and given to the patient to drink. Common flavoring syrups may be added to the solution to improve the taste for oral administration. The diluted solution may be administered via a nasogastric tube.
登录到您的免费帐户