FDA records indicate that there are no current recalls for this drug.
您是医疗专业人员吗?
Trending Topics
Prilosec Recall
获取警报召回时。
Questions & Answers
副作用和不良反应
There is currently no warning information available for this product. We apologize for any inconvenience.
法律问题
目前尚无该药物可用的法律信息。
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
目前还没有生产informa的警告tion available for this drug.
FDA Labeling Changes
目前,该药物尚无FDA标签更改。
Uses
1 INDICATIONS AND USAGE 1.1 Duodenal Ulcer (adults)
奥美拉唑短期治疗的指示active duodenal ulcer in adults. Most patients heal within four weeks. Some patients may require an additional four weeks of therapy.
PRILOSEC in combination with clarithromycin and amoxicillin, is indicated for treatment of patients withH. pyloriinfection and duodenal ulcer disease (active or up to 1-year history) to eradicateH. pyloriin adults.
PRILOSEC, in combination with clarithromycin is indicated for treatment of patients withH. pyloriinfection and duodenal ulcer disease to eradicateH. pyloriin adults.
根除H. pylorihas been shown to reduce the risk of duodenal ulcer recurrence [see Clinical Studies (14.1) and Dosage and Administration (2)].
在治疗失败的患者中,与三重治疗相比,用克拉霉素的Prilosec更可能与克拉霉素耐药性的发展有关。在治疗失败的患者中,应进行敏感性测试。如果证明对克拉霉素的耐药性或不可能进行敏感性测试,则应进行替代性抗菌治疗。[[See Microbiology section (12.4)],以及Clarithromycin包装插入,微生物学部分。
1.2胃溃疡(成人)PRILOSEC is indicated for short-term treatment (4-8 weeks) of active benign gastric ulcer in adults. [参见临床研究(14.2)]
1.3治疗胃食管反流疾病(GERD)(成人和小儿患者)有症状的GERD
PRILOSEC is indicated for the treatment of heartburn and other symptoms associated with GERD in pediatric patients and adults.
侵蚀性食管炎
PRILOSEC is indicated for the short-term treatment (4-8 weeks) of erosive esophagitis that has been diagnosed by endoscopy in pediatric patients and adults. [参见临床研究(14.4)]
The efficacy of PRILOSEC used for longer than 8 weeks in these patients has not been established. If a patient does not respond to 8 weeks of treatment, an additional 4 weeks of treatment may be given. If there is recurrence of erosive esophagitis or GERD symptoms (eg, heartburn), additional 4-8 week courses of omeprazole may be considered.
1.4 Maintenance of Healing of Erosive Esophagitis (adults and pediatric patients)PRILOSEC is indicated to maintain healing of erosive esophagitis in pediatric patients and adults.
Controlled studies do not extend beyond 12 months. [参见临床研究(14.4)]
1.5 Pathological Hypersecretory Conditions (adults)PRILOSEC is indicated for the long-term treatment of pathological hypersecretory conditions (eg, Zollinger-Ellison syndrome, multiple endocrine adenomas and systemic mastocytosis) in adults.
History
There is currently no drug history available for this drug.
其他信息
11 DESCRIPTION
Prilosec(Omeprazole)延迟释放胶囊中的活性成分是一种取代的苯咪唑,5-甲氧基-2- [[((4-甲氧基-3,5-甲氧基-3,5-二甲基-2-吡啶基)甲基]甲基] - 1-1H-benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C17H19N3O3S,分子量为345.42。结构公式是:
STRUCTURE IMAGE
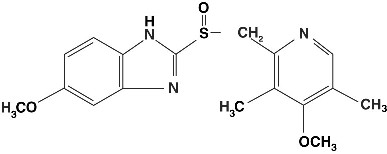
Omeprazole is a white to off-white crystalline powder that melts with decomposition at about 155°C. It is a weak base, freely soluble in ethanol and methanol, and slightly soluble in acetone and isopropanol and very slightly soluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media, but has acceptable stability under alkaline conditions.
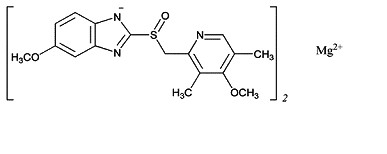
The active ingredient in PRILOSEC (omeprazole magnesium) for Delayed Release Oral Suspension, is 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, magnesium salt (2:1)
Omeprazole magnesium is a white to off white powder with a melting point with degradation at 200°C. The salt is slightly soluble (0.25 mg/ml) in water at 25°C, and it is soluble in methanol. The half-life is highly pH dependent.
The empirical formula for omeprazole magnesium is (C17H18N3O3S)2Mg, the molecular weight is 713.12 and the structural formula is
STRUCTURE IMAGE 2
PRILOSEC is supplied as delayed-release capsules for oral administration. Each delayed-release capsule contains either 10 mg, 20 mg or 40 mg of omeprazole in the form of enteric-coated granules with the following inactive ingredients: cellulose, disodium hydrogen phosphate, hydroxypropyl cellulose, hypromellose, lactose, mannitol, sodium lauryl sulfate and other ingredients. The capsule shells have the following inactive ingredients: gelatin-NF, FD and C Blue #1, FD and C Red #40, D and C Red #28, titanium dioxide, synthetic black iron oxide, isopropanol, butyl alcohol, FD and C Blue #2, D and C Red #7 Calcium Lake, and, in addition, the 10 mg and 40 mg capsule shells also contain D and C Yellow #10.
Each packet of PRILOSEC For Delayed-Release Oral Suspension contains either 2.8 mg or 11.2 mg of omeprazole magnesium (equivalent to 2.5 mg or 10 mg of omeprazole ), in the form of enteric-coated granules with the following inactive ingredients: glyceryl monostearate, hydroxypropyl cellulose, hypromellose, magnesium stearate, methacrylic acid copolymer C, polysorbate, sugar spheres, talc, and triethyl citrate, and also inactive granules. The inactive granules are composed of the following ingredients: citric acid, crospovidone, dextrose, hydroxypropyl cellulose, iron oxide and xantham gum. The omeprazole granules and inactive granules are constituted with water to form a suspension and are given by oral, nasogastric or direct gastric administration.
Sources
Prilosec Manufacturers
-
Stat RX USA LLC
![Prilosec(奥美拉唑镁)胶囊[Stat Rx USA LLC]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Prilosec | Stat Rx Usa Llc
![Prilosec (Omeprazole Magnesium) Capsule [Stat Rx Usa Llc] Prilosec(奥美拉唑镁)胶囊[Stat Rx USA LLC]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
2剂量和给药PRILOSEC Delayed-Release Capsules should be taken before eating. In the clinical trials, antacids were used concomitantly with PRILOSEC.
Patients should be informed that the PRILOSEC Delayed-Release Capsule should be swallowed whole.
对于无法吞咽完整胶囊的患者,可以使用替代性给药选项。[见剂量和管理(2.8)]
2.1 Short-Term Treatment of Active Duodenal UlcerThe recommended adult oral dose of PRILOSEC is 20 mg once daily. Most patients heal within four weeks. Some patients may require an additional four weeks of therapy.
2.2 H. pylori Eradication for the Reduction of the Risk of Duodenal Ulcer RecurrenceTriple Therapy (PRILOSEC/clarithromycin/amoxicillin) — The recommended adult oral regimen is PRILOSEC 20 mg plus clarithromycin 500 mg plus amoxicillin 1000 mg each given twice daily for 10 days. In patients with an ulcer present at the time of initiation of therapy, an additional 18 days of PRILOSEC 20 mg once daily is recommended for ulcer healing and symptom relief.
双重疗法(Prilosec/Clarithromycin) - 推荐的成年口服方案每天为40 mg Prilosec 40毫克加上Clarithromycin 500 mg每天3次,每天3次,持续14天。在启动治疗时出现溃疡的患者中,建议每天额外14天的prilosec 20毫克每天一次治疗一次,以缓解溃疡愈合和症状。
2.3 Gastric Ulcer建议的成年口服剂量每天40毫克持续4-8周。
2.4 Gastroesophageal Reflux Disease (GERD)建议的成年口服剂量用于治疗有症状的GERD患者,无食管病变为20 mg,持续4周。推荐的成年口服剂量用于治疗侵蚀性食管炎患者和伴随GERD症状的患者每天20毫克,持续4至8周。
2.5 Maintenance of Healing of Erosive Esophagitis建议的成人口服剂量每天20毫克。[见临床研究(14.4)]
2.6 Pathological Hypersecretory ConditionsThe dosage of PRILOSEC in patients with pathological hypersecretory conditions varies with the individual patient. The recommended adult oral starting dose is 60 mg once daily. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Doses up to 120 mg three times daily have been administered. Daily dosages of greater than 80 mg should be administered in divided doses. Some patients with Zollinger-Ellison syndrome have been treated continuously with PRILOSEC for more than 5 years.
2.7 Pediatric PatientsFor the treatment of GERD and maintenance of healing of erosive esophagitis, the recommended daily dose for pediatric patients– 1 to 16 years of age is as follows:
以每公斤的基础,在小儿患者中治愈侵蚀性食管炎所需的奥美拉唑剂量大于成人。
可用于无法吞咽完整囊的小儿患者可以使用替代性管理选择[见剂量和给药(2.8)]。
2.8替代管理选项PRILOSEC is available as a delayed-release capsule or as a delayed-release oral suspension.
对于难以吞咽胶囊的患者,可以将prilosec延迟释放胶囊的含量添加到苹果酱中。应将一汤匙的苹果酱添加到一个空碗中,应打开胶囊。胶囊内部的所有颗粒都应小心地在苹果酱上清空。应将颗粒与苹果酱混合,然后立即用一杯凉水吞咽,以确保完全吞咽颗粒。所使用的苹果酱不应该很热,应该足够柔软,可以吞咽而不咀嚼。不应咀嚼或压碎颗粒。颗粒/苹果酱混合物不应存储以备将来使用。
延迟释放口服悬挂的Prilosec应如下管理:
将2.5 mg包装的内容物将其倒入包含5毫升水的容器中。
Empty the contents of a 10 mg packet into a container containing 15 mL of water.
Stir
留下2至3分钟以加厚。
搅拌并在30分钟内喝酒。
If any material remains after drinking, add more water, stir and drink immediately.
For patients with a nasogastric or gastric tube in place:
Add 5 mL of water to a catheter tipped syringe and then add the contents of a 2.5 mg packet (or 15 mL of water for the 10 mg packet). It is important to only use a catheter tipped syringe when administering PRILOSEC through a nasogastric tube or gastric tube.
Immediately shake the syringe and leave 2 to 3 minutes to thicken.
Shake the syringe and inject through the nasogastric or gastric tube, French size 6 or larger, into the stomach within 30 minutes.
Refill the syringe with an equal amount of water.
Shake and flush any remaining contents from the nasogastric or gastric tube into the stomach.
-
Astrazeneca Lp
![Prilosec (Omeprazole Magnesium) Capsule, Delayed Release Prilosec (Omeprazole Magnesium) Granule, Delayed Release [Astrazeneca Lp]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Prilosec | Bryant Ranch Prepack
![Prilosec (Omeprazole Magnesium) Capsule, Delayed Release Prilosec (Omeprazole Magnesium) Granule, Delayed Release [Astrazeneca Lp] Prilosec (Omeprazole Magnesium) Capsule, Delayed Release Prilosec (Omeprazole Magnesium) Granule, Delayed Release [Astrazeneca Lp]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Lansoprazole is available as a capsule in 15 mg and 30 mg strengths. Direction for use specific to the route and available methods of administration of this dosage form is presented below. Lansoprazole should be taken before eating. Lansoprazole delayed-release capsules SHOULD NOT BE CRUSHED OR CHEWED. In the clinical trials, antacids were used concomitantly with lansoprazole.
2.1推荐剂量*请参阅阿莫西林和克拉霉素的全套处方信息,以获取禁忌症和警告,以及有关老年人和肾脏损害患者的给药的信息。
† Controlled studies did not extend beyond indicated duration.
‡ For patients who do not heal with lansoprazole for 8 weeks (5 to 10%), it may be helpful to give an additional 8 weeks of treatment. If there is a recurrence of erosive esophagitis, an additional 8 week course of lansoprazole may be considered.
§The lansoprazole dose was increased (up to 30 mg twice daily) in some pediatric patients after 2 or more weeks of treatment if they remained symptomatic. For pediatric patients unable to swallow an intact capsule please see Administration Options.
¶Varies with individual patient. Recommended adult starting dose is 60 mg once daily. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Dosages up to 90 mg twice daily have been administered. Daily dose of greater than 120 mg should be administered in divided doses. Some patients with Zollinger-Ellison Syndrome have been treated continuously with lansoprazole for more than 4 years.
#Controlled studies did not extend beyond 12 months.
Indication
Recommended Dose
Frequency
Duodenal Ulcers
Short-Term Treatment
15 mg
Once daily for 4 weeks
维护治愈
15 mg
Once daily
H. pylori Eradication to
降低
Duodenal Ulcer Recurrence*
Triple Therapy:
Lansoprazole
30毫克
每天两次(Q12H)10或14天
Amoxicillin
1 gram
每天两次(Q12H)10或14天
克拉霉素
500 mg
每天两次(Q12H)10或14天
Dual Therapy:
Lansoprazole
30毫克
Three times daily (q8h) for 14 days
Amoxicillin
1 gram
Three times daily (q8h) for 14 days
Benign Gastric Ulcer
Short-Term Treatment
30毫克
Once daily for up to 8 weeks
NSAID-associated Gastric Ulcer
Healing
30毫克
每天一次八周†
Risk Reduction
15 mg
Once daily for up to 12 weeks†
Gastroesophageal Reflux Disease (GERD)
Short-Term Treatment of Symptomatic GERD
15 mg
Once daily for up to 8 weeks
Short-Term
侵蚀性食管炎
30毫克
Once daily for up to 8 weeks‡
Pediatric
(1 to 11 years of age)
Short-Term Treatment of Symptomatic GERD and Short-Term Treatment of Erosive Esophagitis
≤ 30 kg
15 mg
Once daily for up to 12 weeks§
> 30公斤
30毫克
Once daily for up to 12 weeks§
(12 to 17 year of age)
Short-Term Treatment of Symptomatic GERD
非弱性GERD
15 mg
Once daily for up to 8 weeks
侵蚀性食管炎
30毫克
Once daily for up to 8 weeks
Maintenance of Healing of Erosive Esophagitis
15 mg
Once daily
Pathological Hypersecretory Conditions including Zollinger-Ellison Syndrome
60 mg
Once daily¶
Patients should be instructed that if a dose is missed, it should be taken as soon as possible. However, if the next scheduled dose is due, the patient should not take the missed dose, and should be instructed to take the next dose on time. Patients should be instructed not to take 2 doses at one time to make up for a missed dose.
2.2 Special PopulationsRenal impairment patients and geriatric patients do not require dosage adjustment. However, consider dose adjustment in patients with severe liver impairment [see USE IN SPECIFIC POPULATIONS (8.5, 8.6 and 8.7)].
2.3 Important Administration InformationAdministration Option
Lansoprazole Delayed-release Capsules – Oral Administration
兰索拉唑延迟释放的胶囊应吞咽整体。或者,对于吞咽胶囊困难的患者,可以打开和给药兰索拉唑延迟释放的胶囊如下:о Open capsule.
请在一汤匙的苹果酱上撒上完整的颗粒,确保布丁,奶酪,酸奶或紧张的梨。
о Swallow immediately.
Lansoprazole delayed-release capsules may also be emptied into a small volume of either apple juice, orange juice or tomato juice and administered as follows:о Open capsule.
о Sprinkle intact granules into a small volume of either apple juice, orange juice or tomato juice (60 mL – approximately 2 ounces).
о Mix briefly.
о Swallow immediately.
为了确保剂量的完全递送,应用两量或更多量的果汁冲洗玻璃,并立即吞咽物品。
兰索拉唑延迟释放胶囊-Nasogastric Tube(≥16法国)给药
For patients who have a nasogastric tube in place, lansoprazole delayed-release capsules can be administered as follows:о Open capsule.
- 将完整的颗粒混合成40毫升苹果汁。不要使用其他液体。
о Inject through the nasogastric tube into the stomach.
о Flush with additional apple juice to clear the tube.
在其他食物和液体中的使用尚未在临床上进行研究,因此不建议使用。
Login To Your Free Account


![Prilosec (Omeprazole Magnesium) Capsule, Delayed Release Prilosec (Omeprazole Magnesium) Granule, Delayed Release [Astrazeneca Lp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=acdb9712-1d1a-4b34-988e-6b4cbb8df9e2&name=56321.jpg)