FDA records indicate that there are no current recalls for this drug.
您是医疗专业人员吗?
Trending Topics
AciphexRecall
获取警报召回时。
Questions & Answers
副作用和不良反应
目前还没有warning information available for this product. We apologize for any inconvenience.
Legal Issues
目前尚无该药物可用的法律信息。
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
目前还没有manufacturer warning information available for this drug.
FDA Labeling Changes
目前,该药物尚无FDA标签更改。
Uses
Enter section text here
Aciphex在短期治疗(4至8周)治疗方面,以侵蚀性或溃疡性胃食管反流疾病(GERD)的愈合和症状缓解。对于那些在治疗8周后未愈合的患者,可以考虑另外8周的ACIPHEX病程。
ACIPHEX表示用于维持侵蚀性或溃疡性胃食管反流疾病(GERD维持)患者的胃灼热症状复发率的愈合和降低。对照研究不会延长超过12个月。
ACIPHEX用于治疗白天和夜间胃灼热以及与成年人和青少年12岁及以上的GERD相关的其他症状。
ACIPHEX is indicated for short-term (up to four weeks) treatment in the healing and symptomatic relief of duodenal ulcers. Most patients heal within four weeks.
ACIPHEX in combination with amoxicillin and clarithromycin as a three drug regimen, is indicated for the treatment of patients with幽门螺杆菌infection and duodenal ulcer disease (active or history within the past 5 years) to eradicate幽门螺杆菌。根除幽门螺杆菌has been shown to reduce the risk of duodenal ulcer recurrence. {SeeCLINICAL STUDIES(14.5) and剂量和给药(2.5)}
在治疗失败的患者中,应进行敏感性测试。如果证明对克拉霉素的耐药性或不可能进行敏感性测试,则应进行替代性抗菌治疗。{看CLINICAL PHARMACOLOGY, Microbiology(12.2) and the clarithromycin package insert,CLINICAL PHARMACOLOGY, Microbiology}
o ACIPHEX表示长期治疗f pathological hypersecretory conditions, including Zollinger-Ellison syndrome.
History
目前还没有drug history available for this drug.
其他信息
The active ingredient in ACIPHEX Delayed-Release Tablets is rabeprazole sodium, a substituted benzimidazole that inhibits gastric acid secretion. Rabeprazole sodium is known chemically as 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole sodium salt. It has an empirical formula of C18H20N3Nao3S and a molecular weight of 381.43. Rabeprazole sodium is a white to slightly yellowish-white solid. It is very soluble in water and methanol, freely soluble in ethanol, chloroform and ethyl acetate and insoluble in ether and n-hexane. The stability of rabeprazole sodium is a function of pH; it is rapidly degraded in acid media, and is more stable under alkaline conditions. The structural formula is:
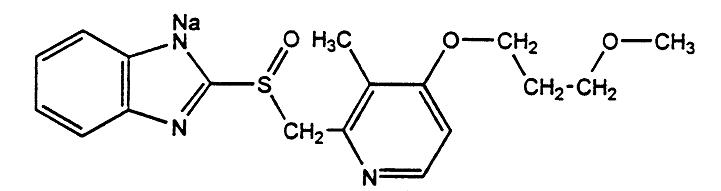
Aciphex可作为延迟释放的,含有20毫克的Rabeprazole钠的肠涂层片。
Inactive ingredients of the 20 mg tablet are carnauba wax, crospovidone, diacetylated monoglycerides, ethylcellulose, hydroxypropyl cellulose, hypromellose phthalate, magnesium stearate, mannitol, propylene glycol, sodium hydroxide, sodium stearyl fumarate, talc, and titanium dioxide. Iron oxide yellow is the coloring agent for the tablet coating. Iron oxide red is the ink pigment.
Sources
Aciphex制造商
-
医生总保健公司。
![Aciphex(Rabeprazole Sodium) Tablet, Delayed Release [Physicians Total Care, Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Aciphex |医生总保健公司。
![Aciphex (Rabeprazole Sodium) Tablet, Delayed Release [Physicians Total Care, Inc.] Aciphex(Rabeprazole Sodium) Tablet, Delayed Release [Physicians Total Care, Inc.]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
ACIPHEX tablets should be swallowed whole. The tablets should not be chewed, crushed, or split. ACIPHEX can be taken with or without food.
2.1 Healing of Erosive or Ulcerative GERD推荐的成人口服剂量是一个Aciphex 20毫克延迟释放片,每天服用一次四到八周{参见指示和用法。(1.1)}。对于那些在治疗8周后未愈合的患者,可以考虑另外8周的ACIPHEX病程。
2.2 Maintenance of Healing of Erosive or Ulcerative GERD建议的成人口服剂量是每天服用一次20毫克延迟释放片剂。{请参阅指示和用法(1.2)}。
2.3有症状的GERD治疗The recommended adult oral dose is one ACIPHEX 20 mg delayed-release tablet to be taken once daily for 4 weeks. {See INDICATIONS AND USAGE (1.3)} If symptoms do not resolve completely after 4 weeks, an additional course of treatment may be considered. The recommended adolescent dosing is listed in Section 2.7.
2.4 Healing of Duodenal Ulcers推荐的成年口服剂量是一杯20毫克延迟释放片,每天在早饭后每天服用一次,持续四个星期。{请参阅指示和用法(1.4)}。大多数十二指肠溃疡患者在四个星期内愈合。一些患者可能需要额外的治疗才能实现康复。
2.5 Helicobacter pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence TABLE 1 THREE DRUG REGIMENa: Aciphex 20 mg Twice Daily for 7 Days Amoxicillin 1000 mg Twice Daily for 7 Days Clarithromycin 500 mg Twice Daily for 7 Days All three medications should be taken twice daily with the morning and evening meals.
A很重要的是,患者遵守完整的7天治疗方案。{看CLINICAL STUDIES section.(14.5)}.
2.6 Treatment of Pathological Hypersecretory Conditions Including Zollinger-Ellison SyndromeThe dosage of ACIPHEX in patients with pathologic hypersecretory conditions varies with the individual patient. The recommended adult oral starting dose is 60 mg once a day. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Some patients may require divided doses. Doses up to 100 mg QD and 60 mg BID have been administered. Some patients with Zollinger-Ellison syndrome have been treated continuously with ACIPHEX for up to one year.
2.7 Short-term Treatment of GERD in Adolescent Patients 12 Years of Age and Above12岁及以上青少年的建议口服剂量每天每天20毫克,最多8周{参见儿科使用(8.4)}。
2.8 Elderly, Renal and Hepatic Impaired PatientsNo dosage adjustment is necessary in elderly patients, in patients with renal disease or in patients with mild to moderate hepatic impairment. Administration of rabeprazole to patients with mild to moderate liver impairment resulted in increased exposure and decreased elimination. Due to the lack of clinical data on rabeprazole in patients with severe hepatic impairment, caution should be exercised in those patients.
-
Lake Erie Medical & Surgical Supply Dba Quality Care Produtcs Llc
![Aciphex(Rabeprazole钠)片剂,涂层[伊利湖医疗和外科供应DBA质量护理Produtcs LLC]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Aciphex |Lake Erie Medical & Surgical Supply Dba Quality Care Produtcs Llc
![Aciphex (Rabeprazole Sodium) Tablet, Coated [Lake Erie Medical & Surgical Supply Dba Quality Care Produtcs Llc] Aciphex(Rabeprazole钠)片剂,涂层[伊利湖医疗和外科供应DBA质量护理Produtcs LLC]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
ACIPHEX tablets should be swallowed whole. The tablets should not be chewed, crushed, or split. ACIPHEX can be taken with or without food.
2.1。侵蚀或溃疡性GERD的治愈The recommended adult oral dose is one ACIPHEX 20 mg delayed-release tablet to be taken once daily for four to eight weeks. {See INDICATIONS AND USAGE (1.1)}. For those patients who have not healed after 8 weeks of treatment, an additional 8-week course of ACIPHEX may be considered.
2.2。维持侵蚀或溃疡性GERD的治愈建议的成人口服剂量是每天服用一次20毫克延迟释放片剂。{请参阅指示和用法(1.2)}。
2.3. Treatment of Symptomatic GERDThe recommended adult oral dose is one ACIPHEX 20 mg delayed-release tablet to be taken once daily for 4 weeks. {See INDICATIONS AND USAGE (1.3)}. If symptoms do not resolve completely after 4 weeks, an additional course of treatment may be considered. The recommended adolescent dosing is listed in Section 2.7.
2.4。十二指肠溃疡的愈合推荐的成年口服剂量是一杯20毫克延迟释放片,每天在早饭后每天服用一次,持续四个星期。{请参阅指示和用法(1.4)}。大多数十二指肠溃疡患者在四个星期内愈合。一些患者可能需要额外的治疗才能实现康复。
2.5. Helicobacter pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence TABLE 1 THREE DRUG REGIMENa All three medications should be taken twice daily with the morning and evening meals.
A很重要的是,患者遵守完整的7天治疗方案。{参见临床研究部分(14.5)}。ACIPHEX 20 mg Twice Daily for 7 Days Amoxicillin 1000 mg Twice Daily for 7 Days Clarithromycin 500 mg Twice Daily for 7 Days2.6. Treatment of Pathological Hypersecretory Conditions, Including Zollinger-Ellison SyndromeThe dosage of ACIPHEX in patients with pathologic hypersecretory conditions varies with the individual patient. The recommended adult oral starting dose is 60 mg once a day. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Some patients may require divided doses. Doses up to 100 mg QD and 60 mg BID have been administered. Some patients with Zollinger-Ellison syndrome have been treated continuously with ACIPHEX for up to one year.
2.7. Short-term Treatment of GERD in Adolescent Patients 12 Years of Age and Above12岁及以上青少年的建议口服剂量每天每天20毫克,最多8周{参见儿科使用(8.4)}。
2.8。老年人,肾脏和肝障碍患者No dosage adjustment is necessary in elderly patients, in patients with renal disease or in patients with mild to moderate hepatic impairment. Administration of rabeprazole to patients with mild to moderate liver impairment resulted in increased exposure and decreased elimination. Due to the lack of clinical data on rabeprazole in patients with severe hepatic impairment, caution should be exercised in those patients.
-
伊利湖医疗和外科用品DBA质量护理产品有限责任公司
![Aciphex(Rabeprazole Sodium) Tablet, Coated [Lake Erie Medical & Surgical Supplies Dba Quality Care Products Llc]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Aciphex |伊利湖医疗和外科用品DBA质量护理产品有限责任公司
![Aciphex (Rabeprazole Sodium) Tablet, Coated [Lake Erie Medical & Surgical Supplies Dba Quality Care Products Llc] Aciphex(Rabeprazole Sodium) Tablet, Coated [Lake Erie Medical & Surgical Supplies Dba Quality Care Products Llc]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
ACIPHEX tablets should be swallowed whole. The tablets should not be chewed, crushed, or split. ACIPHEX can be taken with or without food.
2.1。侵蚀或溃疡性GERD的治愈The recommended adult oral dose is one ACIPHEX 20 mg delayed-release tablet to be taken once daily for four to eight weeks. {See INDICATIONS AND USAGE (1.1)}. For those patients who have not healed after 8 weeks of treatment, an additional 8-week course of ACIPHEX may be considered.
2.2。维持侵蚀或溃疡性GERD的治愈建议的成人口服剂量是每天服用一次20毫克延迟释放片剂。{请参阅指示和用法(1.2)}。
2.3. Treatment of Symptomatic GERDThe recommended adult oral dose is one ACIPHEX 20 mg delayed-release tablet to be taken once daily for 4 weeks. {See INDICATIONS AND USAGE (1.3)}. If symptoms do not resolve completely after 4 weeks, an additional course of treatment may be considered. The recommended adolescent dosing is listed in Section 2.7.
2.4。十二指肠溃疡的愈合推荐的成年口服剂量是一杯20毫克延迟释放片,每天在早饭后每天服用一次,持续四个星期。{请参阅指示和用法(1.4)}。大多数十二指肠溃疡患者在四个星期内愈合。一些患者可能需要额外的治疗才能实现康复。
2.5. Helicobacter pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence TABLE 1 THREE DRUG REGIMENa All three medications should be taken twice daily with the morning and evening meals.
A很重要的是,患者遵守完整的7天治疗方案。{参见临床研究部分(14.5)}。ACIPHEX 20 mg Twice Daily for 7 Days Amoxicillin 1000 mg Twice Daily for 7 Days Clarithromycin 500 mg Twice Daily for 7 Days2.6. Treatment of Pathological Hypersecretory Conditions, Including Zollinger-Ellison SyndromeThe dosage of ACIPHEX in patients with pathologic hypersecretory conditions varies with the individual patient. The recommended adult oral starting dose is 60 mg once a day. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Some patients may require divided doses. Doses up to 100 mg QD and 60 mg BID have been administered. Some patients with Zollinger-Ellison syndrome have been treated continuously with ACIPHEX for up to one year.
2.7. Short-term Treatment of GERD in Adolescent Patients 12 Years of Age and Above12岁及以上青少年的建议口服剂量每天每天20毫克,最多8周{参见儿科使用(8.4)}。
2.8。老年人,肾脏和肝障碍患者No dosage adjustment is necessary in elderly patients, in patients with renal disease or in patients with mild to moderate hepatic impairment. Administration of rabeprazole to patients with mild to moderate liver impairment resulted in increased exposure and decreased elimination. Due to the lack of clinical data on rabeprazole in patients with severe hepatic impairment, caution should be exercised in those patients.
-
红衣主教健康
![Aciphex(Rabeprazole钠)片剂,涂层[Cardinal Health]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Aciphex |红衣主教健康
![Aciphex (Rabeprazole Sodium) Tablet, Coated [Cardinal Health] Aciphex(Rabeprazole钠)片剂,涂层[Cardinal Health]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
ACIPHEX tablets should be swallowed whole. The tablets should not be chewed, crushed, or split. ACIPHEX can be taken with or without food.
2.1 Healing of Erosive or Ulcerative GERDThe recommended adult oral dose is one ACIPHEX 20 mg delayed-release tablet to be taken once daily for four to eight weeks. {See INDICATIONS AND USAGE (1.1)}. For those patients who have not healed after 8 weeks of treatment, an additional 8-week course of ACIPHEX may be considered.
2.2 Maintenance of Healing of Erosive or Ulcerative GERD建议的成人口服剂量是每天服用一次20毫克延迟释放片剂。{请参阅指示和用法(1.2)}。
2.3有症状的GERD治疗The recommended adult oral dose is one ACIPHEX 20 mg delayed-release tablet to be taken once daily for 4 weeks. {See INDICATIONS AND USAGE (1.3)}. If symptoms do not resolve completely after 4 weeks, an additional course of treatment may be considered. The recommended adolescent dosing is listed in Section 2.7.
2.4 Healing of Duodenal Ulcers推荐的成年口服剂量是一杯20毫克延迟释放片,每天在早饭后每天服用一次,持续四个星期。{请参阅指示和用法(1.4)}。大多数十二指肠溃疡患者在四个星期内愈合。一些患者可能需要额外的治疗才能实现康复。
2.5 Helicobacter pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence TABLE 1 THREE DRUG REGIMEN a All three medications should be taken twice daily with the morning and evening meals.
A很重要的是,患者遵守完整的7天治疗方案。{参见临床研究部分(14.5)}。Aciphex每天两次20毫克阿莫西林每天两次1000毫克每天两次克拉霉素500毫克每天两次500毫克,持续7天2.6治疗病理性超注重疾病,包括Zollinger-Ellison综合征The dosage of ACIPHEX in patients with pathologic hypersecretory conditions varies with the individual patient. The recommended adult oral starting dose is 60 mg once a day. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Some patients may require divided doses. Doses up to 100 mg QD and 60 mg BID have been administered. Some patients with Zollinger-Ellison syndrome have been treated continuously with ACIPHEX for up to one year.
2.7 Short-term Treatment of GERD in Adolescent Patients 12 Years of Age and Above12岁及以上青少年的建议口服剂量每天每天20毫克,最多8周{参见儿科使用(8.4)}。
2.8 Elderly, Renal and Hepatic Impaired PatientsNo dosage adjustment is necessary in elderly patients, in patients with renal disease or in patients with mild to moderate hepatic impairment. Administration of rabeprazole to patients with mild to moderate liver impairment resulted in increased exposure and decreased elimination. Due to the lack of clinical data on rabeprazole in patients with severe hepatic impairment, caution should be exercised in those patients.
-
Bryant Ranch Prepack
![Aciphex(Rabeprazole钠)片剂,涂层[Bryant Ranch Prepack]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Aciphex |Bryant Ranch Prepack
![Aciphex (Rabeprazole Sodium) Tablet, Coated [Bryant Ranch Prepack] Aciphex(Rabeprazole钠)片剂,涂层[Bryant Ranch Prepack]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
ACIPHEX tablets should be swallowed whole. The tablets should not be chewed, crushed, or split. ACIPHEX can be taken with or without food.
2.1 Healing of Erosive or Ulcerative GERDThe recommended adult oral dose is one ACIPHEX 20 mg delayed-release tablet to be taken once daily for four to eight weeks. {See INDICATIONS AND USAGE (1.1)}. For those patients who have not healed after 8 weeks of treatment, an additional 8-week course of ACIPHEX may be considered.
2.2 Maintenance of Healing of Erosive or Ulcerative GERD建议的成人口服剂量是每天服用一次20毫克延迟释放片剂。{请参阅指示和用法(1.2)}。
2.3有症状的GERD治疗The recommended adult oral dose is one ACIPHEX 20 mg delayed-release tablet to be taken once daily for 4 weeks. {See INDICATIONS AND USAGE (1.3)}. If symptoms do not resolve completely after 4 weeks, an additional course of treatment may be considered. The recommended adolescent dosing is listed in Section 2.7.
2.4 Healing of Duodenal Ulcers推荐的成年口服剂量是一杯20毫克延迟释放片,每天在早饭后每天服用一次,持续四个星期。{请参阅指示和用法(1.4)}。大多数十二指肠溃疡患者在四个星期内愈合。一些患者可能需要额外的治疗才能实现康复。
2.5 Helicobacter pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence TABLE 1 THREE DRUG REGIMEN a All three medications should be taken twice daily with the morning and evening meals.
A很重要的是,患者遵守完整的7天治疗方案。{参见临床研究部分(14.5)}。ACIPHEX Delayed Release Tablets 20 mg Twice Daily for 7 Days Amoxicillin 1000 mg Twice Daily for 7 Days Clarithromycin 500 mg Twice Daily for 7 Days 2.6 Treatment of Pathological Hypersecretory Conditions, Including Zollinger-Ellison SyndromeThe dosage of ACIPHEX in patients with pathologic hypersecretory conditions varies with the individual patient. The recommended adult oral starting dose is 60 mg once a day. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Some patients may require divided doses. Doses up to 100 mg QD and 60 mg BID have been administered. Some patients with Zollinger-Ellison syndrome have been treated continuously with ACIPHEX for up to one year.
2.7 Short-term Treatment of GERD in Adolescent Patients 12 Years of Age and Above12岁及以上青少年的建议口服剂量每天每天20毫克,最多8周{参见儿科使用(8.4)}。
2.8 Elderly, Renal and Hepatic Impaired PatientsNo dosage adjustment is necessary in elderly patients, in patients with renal disease or in patients with mild to moderate hepatic impairment. Administration of rabeprazole to patients with mild to moderate liver impairment resulted in increased exposure and decreased elimination. Due to the lack of clinical data on rabeprazole in patients with severe hepatic impairment, caution should be exercised in those patients.
-
Carilion Materials Management
![Aciphex(Rabeprazole Sodium) Tablet, Delayed Release [Carilion Materials Management]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
Aciphex |Carilion Materials Management
![Aciphex (Rabeprazole Sodium) Tablet, Delayed Release [Carilion Materials Management] Aciphex(Rabeprazole Sodium) Tablet, Delayed Release [Carilion Materials Management]](//www.lbxkm.com/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Healing of Erosive or Ulcerative GERD in Adults推荐的成年口服剂量是一个Aciphex 20 mg延迟释放片,每天服用一次四到八周[]。对于那些在治疗8周后未愈合的患者,可以考虑另外8周的ACIPHEX病程。请参阅指示和用法()1.1
2.2 Maintenance of Healing of Erosive or Ulcerative GERD in AdultsThe recommended adult oral dose is one ACIPHEX 20 mg Delayed-Release tablet to be taken once daily [ ]. see Indications and Usage ( ) 1.2
2.3有症状的GERD治疗in Adults推荐的成年口服剂量为一个Aciphex 20 mg延迟释放片,每天服用一次4周[]。如果症状在4周后无法完全缓解,则可以考虑额外的治疗过程。推荐的青少年给药是每天服用一次20毫克延迟释放片剂,持续8周。请参阅指示和用法()1.3
2.4成人十二指肠溃疡的愈合The recommended adult oral dose is one ACIPHEX 20 mg Delayed-Release tablet to be taken once daily after the morning meal for a period up to four weeks [ ]. Most patients with duodenal ulcer heal within four weeks. A few patients may require additional therapy to achieve healing. see Indications and Usage ( ) 1.5
2.5 Eradication to Reduce the Risk of Duodenal Ulcer Recurrence in Adults Helicobacter pylori TABLE 1 THREE DRUG REGIMEN a All three medications should be taken twice daily with the morning and evening meals. It is important that patients comply with the full 7-day regimen [ ].
asee Clinical Studies ( ) 14.5 ACIPHEX Delayed-Release Tablet
20 mg Twice Daily for 7 Days Amoxicillin 1000 mg Twice Daily for 7 Days Clarithromycin 500 mg Twice Daily for 7 Days 2.6 Treatment of Pathological Hypersecretory Conditions, Including Zollinger-Ellison Syndrome in AdultsThe dosage of ACIPHEX in patients with pathologic hypersecretory conditions varies with the individual patient. The recommended adult oral starting dose is 60 mg once daily. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Some patients may require divided doses. Doses up to 100 mg QD and 60 mg BID have been administered. Some patients with Zollinger-Ellison syndrome have been treated continuously with ACIPHEX for up to one year.
2.7 Short-term Treatment of Symptomatic GERD in Adolescent Patients 12 Years of Age and Older12岁及以上的青少年的建议口服剂量是每天延迟释放的片剂,每天一次延迟一次,持续8周。请参阅特定人群中的使用()8.4临床研究()14.7
2.8 Treatment of GERD in Pediatric Patients 1 to 11 Years of AgeThe recommended dosage of ACIPHEX Sprinkle for pediatric patients 1 to 11 years of age by body weight is:
Less than 15 kg: 5 mg once daily for up to 12 weeks with the option to increase to 10 mg if inadequate response [ ]. see Clinical Studies ( ) 14.7 15 kg or more: 10 mg once daily for up to 12 weeks [ ]. see Clinical Studies ( ) 14.7 2.9 Elderly, Renal and Hepatic Impaired PatientsNo dosage adjustment is necessary in elderly patients, in patients with renal disease or in patients with mild to moderate hepatic impairment. Administration of rabeprazole to patients with mild to moderate liver impairment resulted in increased exposure and decreased elimination. Due to the lack of clinical data on rabeprazole in patients with severe hepatic impairment, caution should be exercised in those patients.
2.10政府建议表2高级stration Recommendations Formulation Population Instructions Delayed-Release Tablet Adults and adolescents 12 years of age and older Swallow tablets whole. Do not chew, crush or split tablets. Tablets can be taken with or without food.
Delayed-Release Capsule Pediatric patients 1 to 11 years of age The dose should be taken 30 minutes before a meal. The granules should not be chewed or crushed. Open capsule and sprinkle entire contents on a small amount of soft food (e.g. applesauce, fruit or vegetable based baby food, or yogurt) or empty contents into a small amount of liquid (e.g. infant formula, apple juice, or pediatric electrolyte solution). The whole dose should be taken within 15 minutes of preparation. Food or liquid should be at or below room temperature. Do not store mixture for future use.
-
Eisai Inc.
Login To Your Free Account


![Aciphex(Rabeprazole钠)片剂,涂层[伊利湖医疗和外科供应DBA质量护理Produtcs LLC]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0eed7378-67a4-4b08-aeea-32471ce2028e&name=AcipHex20mgEisai.jpg)
![Aciphex(Rabeprazole Sodium) Tablet, Coated [Lake Erie Medical & Surgical Supplies Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6e6f6c6d-4826-4817-90ff-5c3f4fbe8909&name=AcipHex20mgEisai.jpg)
![Aciphex(Rabeprazole钠)片剂,涂层[Cardinal Health]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=52459f70-e1f5-41bb-a9f4-e68ef5f5dcf5&name=83cfa7e2-3375-469d-bf5c-e77129300329-06.jpg)
![Aciphex(Rabeprazole钠)片剂,涂层[Bryant Ranch Prepack]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c03c0f10-4bd6-4181-9c2a-99facaa0ab1c&name=27331.jpg)
![Aciphex(Rabeprazole Sodium) Tablet, Delayed Release [Carilion Materials Management]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=001dd372-2692-4d6f-a71c-95e7056349a9&name=68151-3834.jpg)
![Aciphex(Rabeprazole Sodium) Tablet, Delayed Release [Eisai Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f5c6a7a2-ec5a-43b0-b819-263ebad66cfe&name=Integra90.jpg)